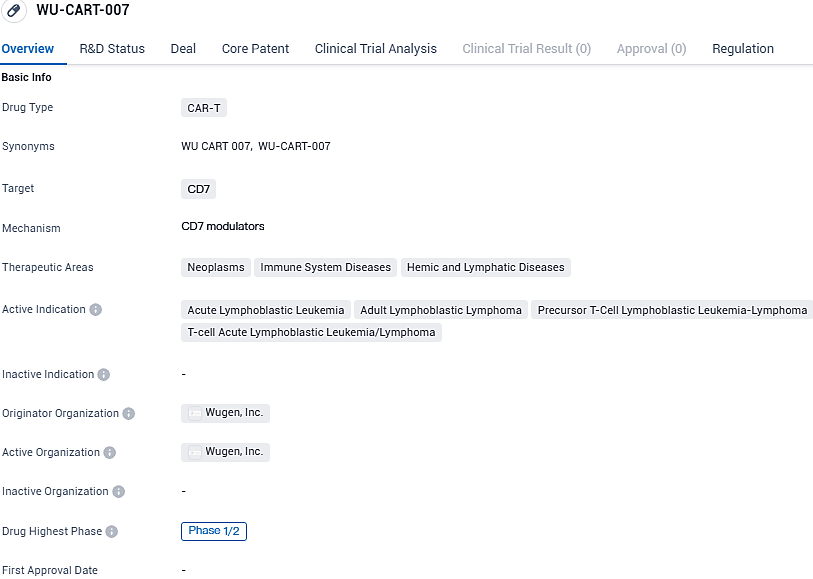
Wugen Reveals Early Data on WU-CART-007 for Tough Blood Cancers at Annual American Hematology Conference
Wugen, Inc., a biotech firm currently in the clinical trial phase, has been working on creating universally-compatible, ready-to-use cellular treatments aimed at addressing blood-related cancers as well as solid tumor cancers. The company recently presented fresh findings from the preliminary Phase 1/2 study focused on identifying the right dosing for WU-CART-007, which is still in ongoing, progressive testing.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Researchers are examining WU-CART-007, an innovative allogeneic CAR-T cell therapy, for its potential in treating individuals with relapsed or refractory T-cell Acute Lymphoblastic Leukemia/Lymphoblastic Lymphoma.
Those facing relapsed or refractory T-ALL have poor outcomes, with about 30% of patients not responding to initial standard therapies. Moreover, of those who initially respond, around half experience a relapse.
Dr. Armin Ghobadi, Associate Professor of Medicine and the Clinical Director at the Washington University's Center for Gene and Cellular Immunotherapy within the Division of Medical Oncology, expressed the urgency in finding improved treatments for a disease that primarily impacts the youth. He noted the encouraging signs of progress, with the expansion of the study yielding promising tolerability and efficacy in treating these challenging blood cancers.
The encouraging results from the initial stages of the WU-CART-007 Phase 1/2 clinical trial highlight the therapy’s prospective role in fulfilling the unmet needs in the treatment of complex blood cancers as it progresses into the next crucial stage of its development. The preliminary data suggest that WU-CART-007 could represent a groundbreaking and efficacious treatment strategy, offering hope for better outcomes in a demographic with limited treatment options.
WU-CART-007 is a novel allogeneic, ready-to-use, fratricide-resistant, CD7-specific CAR-T cell therapy designed to address the complex hurdles associated with deploying CAR-T cells against CD7-expressing hematological malignancies.
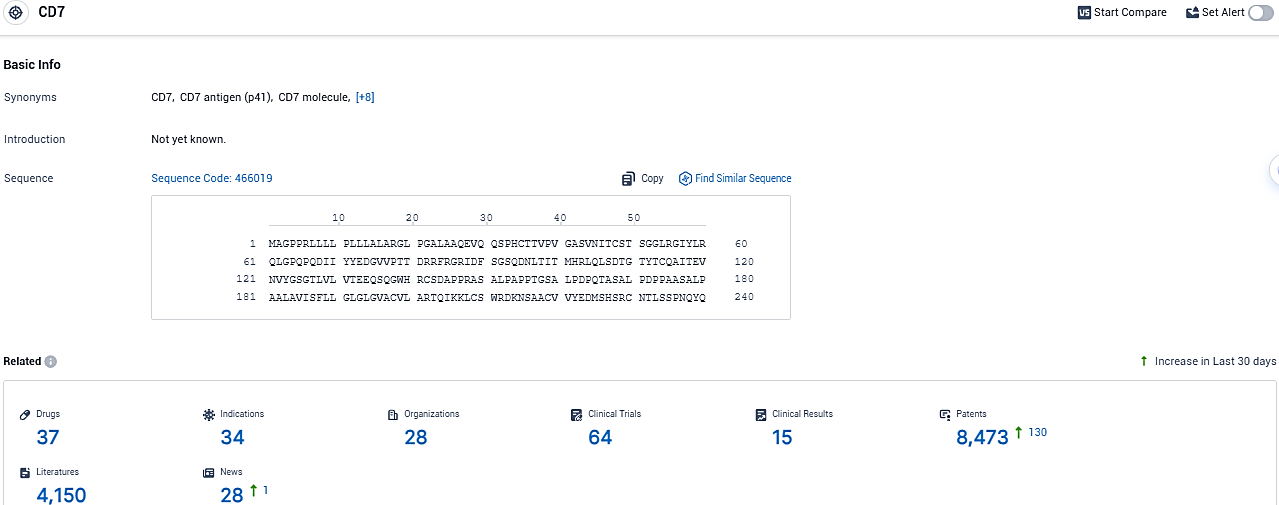
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 19, 2023, there are 37 investigational drugs for the CD7 target, including 34 indications, 28 R&D institutions involved, with related clinical trials reaching 64, and as many as 8473 patents.
WU-CART-007 is manufactured using healthy donor-derived T-cells to eliminate the risk of malignant cell contamination historically observed in the autologous CAR-T setting. WU-CART-007 has received Orphan Drug, Fast Track, and Rare Pediatric Disease Designations from the U.S. Food and Drug Administration for the treatment of R/R T-ALL/LBL.