Zealand: Latest Developments in the Insulin Analog Petrelintide for Weight Management
Recently, the Danish pharmaceutical company Zealand Pharma announced clinical Phase 1 data for its long-acting amylin analogue, Petrelintide, which is being studied for weight management.
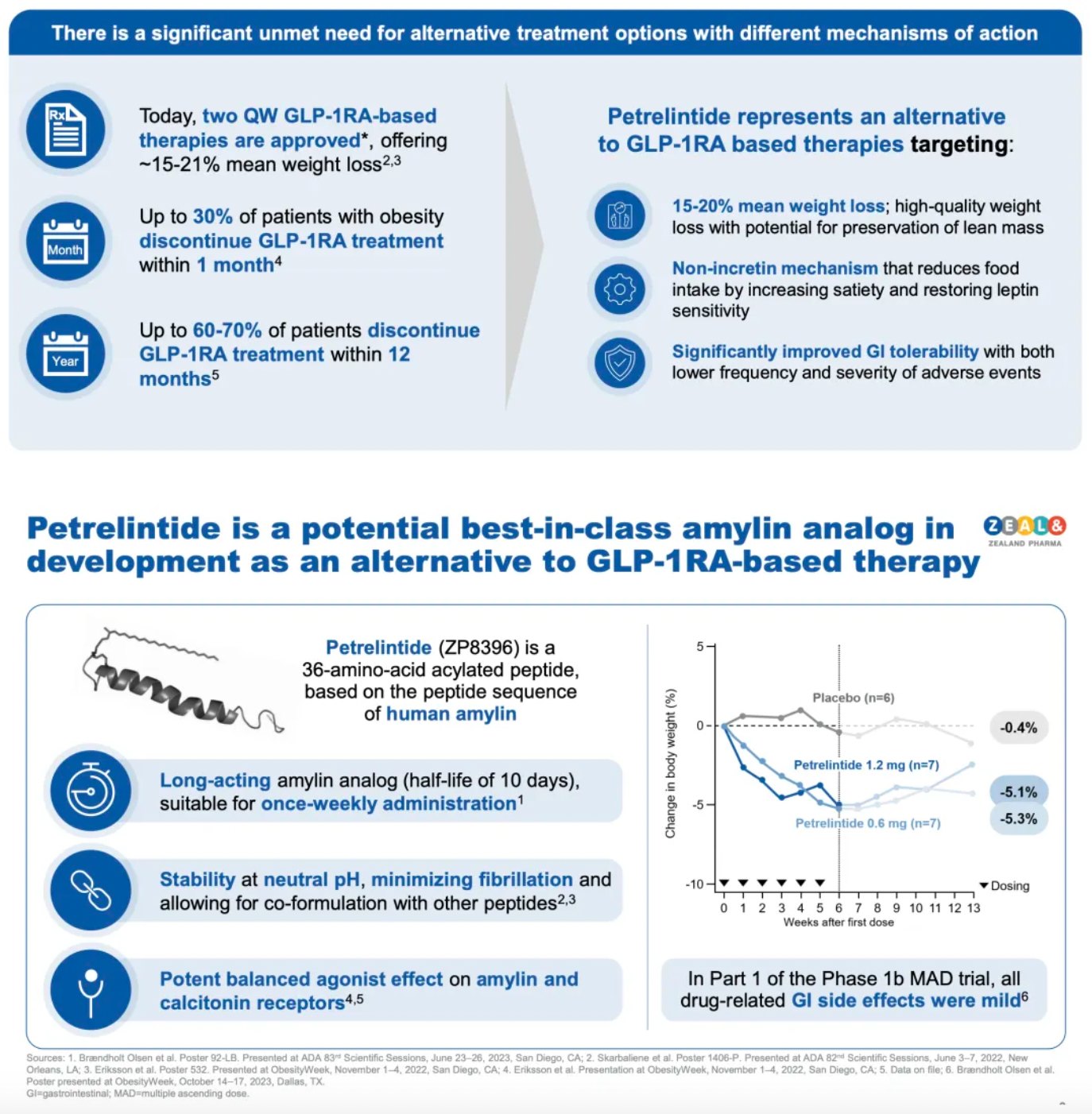
According to statistics mentioned by Zealand, although two approved once-weekly GLP-1 receptor agonists (semaglutide and tirzepatide) have been authorized for treating obesity and can achieve a weight reduction of approximately 15-21%, up to 30% of obese patients discontinue the treatment within the first month of starting a GLP-1 receptor agonist (GLP-1RA) therapy; furthermore, 60-70% of patients stop treatment within the first 12 months. The once-weekly amylin analogue, Petrelintide, may serve as an alternative with a different weight reduction mechanism to GLP-1RA.
Amylin is produced in pancreatic β cells and is co-secreted with insulin following the intake of nutrients. Amylin analogues have been proven to increase satiety by direct action on amylin receptors and by restoring sensitivity to the hormone leptin. This mechanism is distinct from that of GLP-1 receptor agonists, which primarily reduce weight by decreasing appetite.
Petrelintide is a long-acting amylin analogue in development for once-weekly subcutaneous administration. It possesses chemical and physical stability at neutral pH values, minimizes fibrosis, and allows co-formulation with other peptides.
Current clinical or preclinical data suggest that long-acting amylin analogues have the potential to achieve weight loss equivalent to that of GLP-1 receptor agonists but with better tolerability, providing a better experience for patients and achieving high-quality weight loss by preserving lean muscle mass.
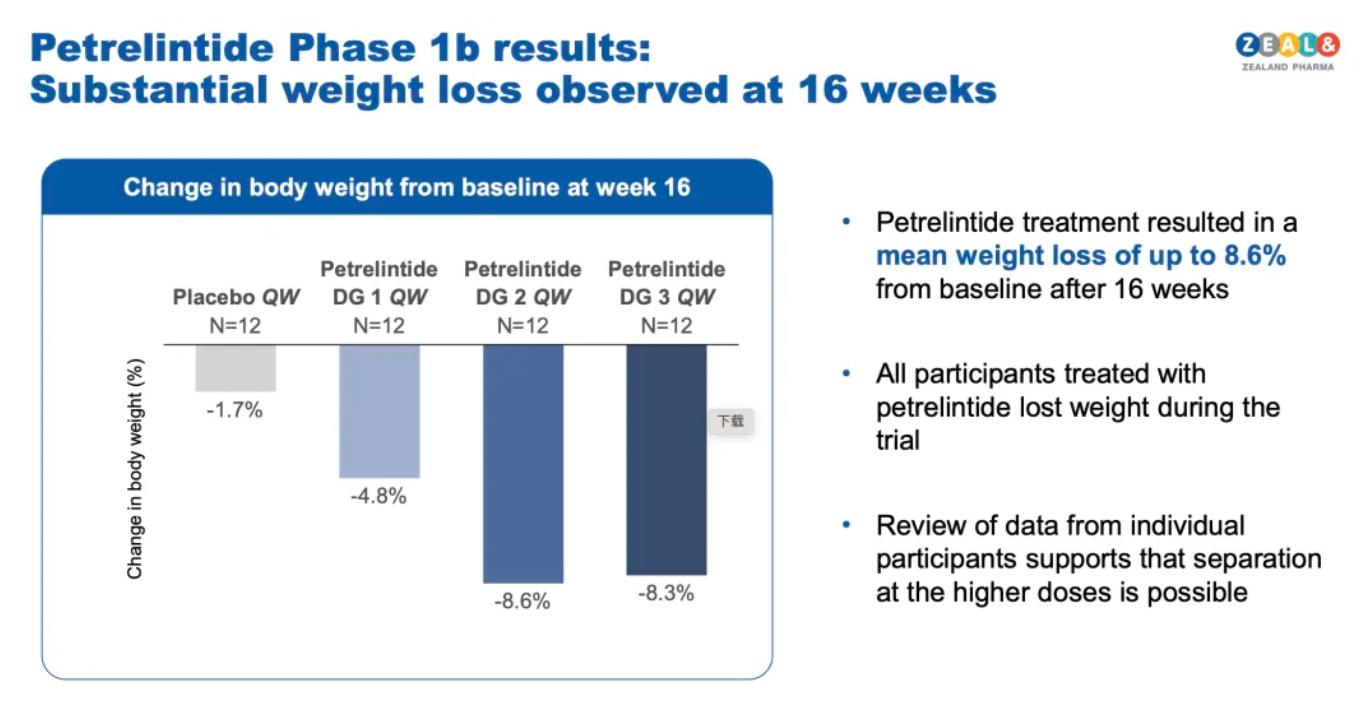
According to the latest clinical trial results, Petrelintide has shown positive weight reduction effects and good tolerability. In a 16-week multiple ascending dose (MAD) trial, participants treated with high doses of Petrelintide experienced an average weight loss of 8.6%, while the placebo group saw only a 1.7% reduction.
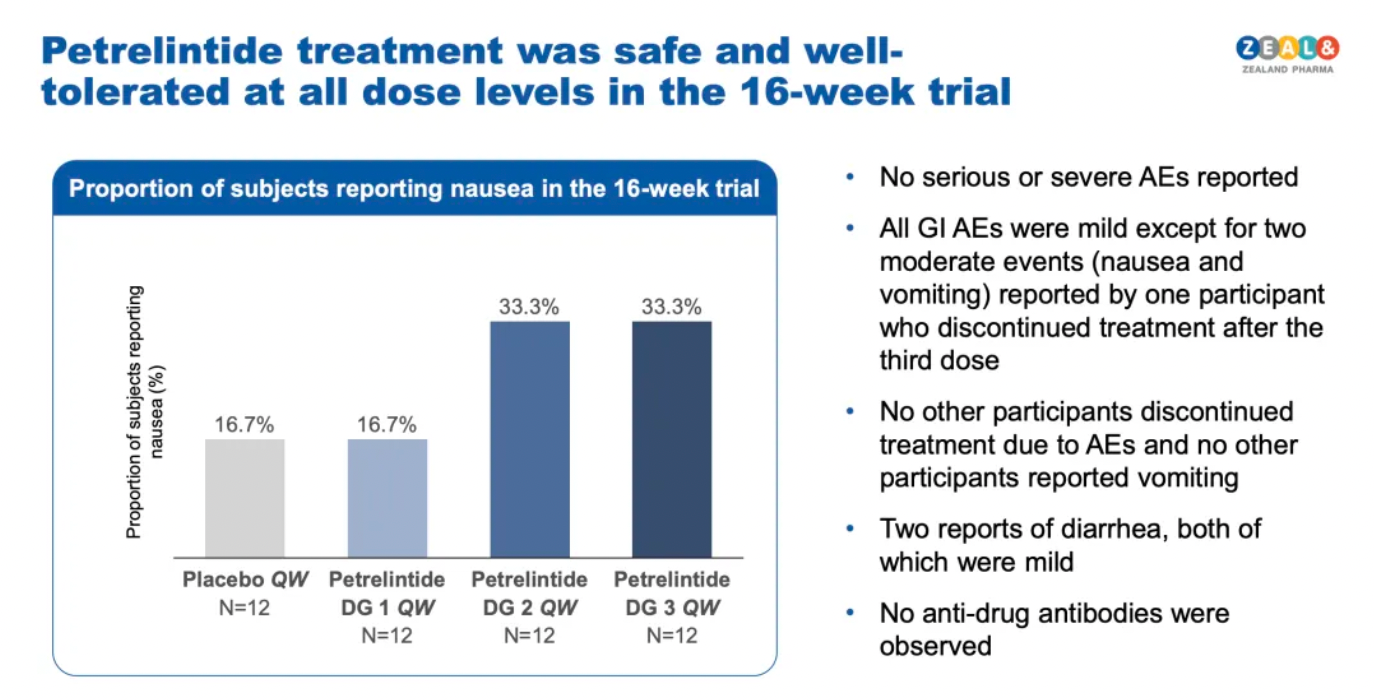
In terms of safety, Petrelintide was considered safe and well-tolerated at all dosage levels, with no serious adverse events reported.
All gastrointestinal adverse events (GI AEs) were mild, except for one participant who reported two moderate events (nausea and vomiting) and discontinued treatment after the third administration. No other participants discontinued treatment due to adverse events, nor were there any other reports of vomiting, with two instances of mild diarrhea also reported.
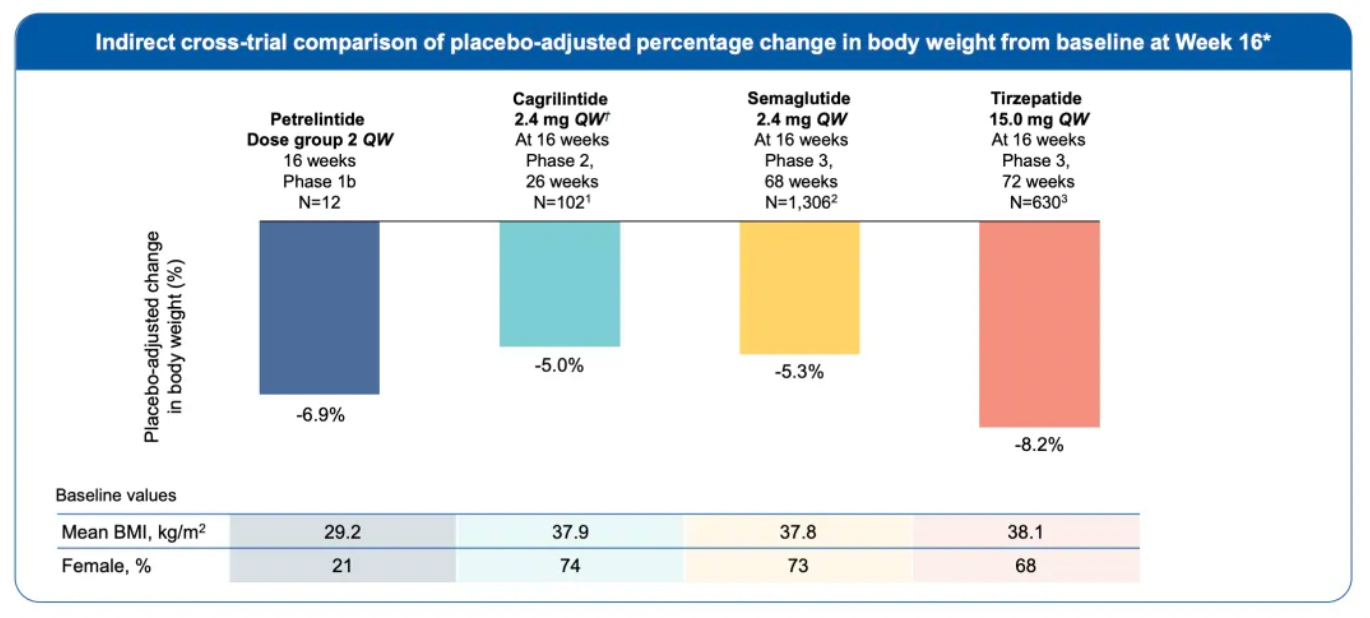
Furthermore, Zealand Pharma also conducted an indirect cross-trial comparison at week 16, comparing Petrelintide with another insulin analog, Cagrilintide (Novo Nordisk), and several GLP-1 receptor agonist (GLP-1RA) products, demonstrating Petrelintide's potential as the best-in-class (BIC) treatment.
Comparative data:
Cagrilintide: Achieved a 5.0% weight reduction in a 26-week Phase 2 clinical trial.
Semaglutide: Achieved a 5.3% weight reduction in a 68-week Phase 3 clinical trial.
Tirzepatide: Achieved an 8.2% weight reduction in a 72-week Phase 3 clinical trial.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!