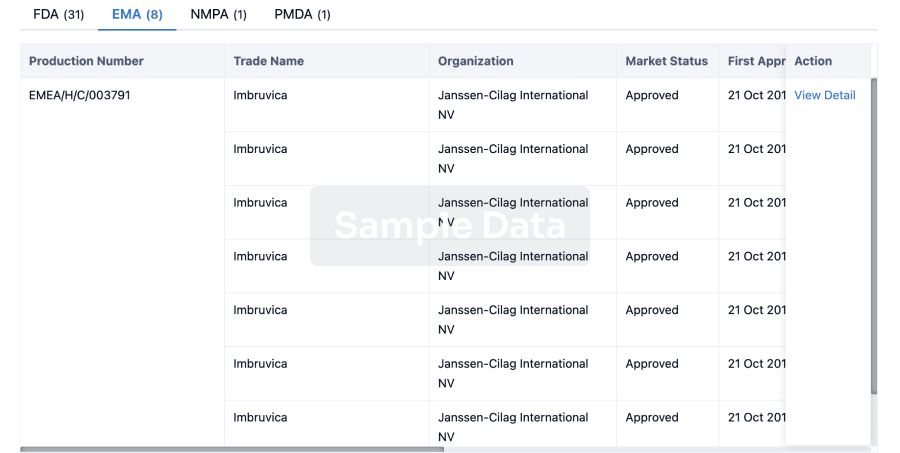
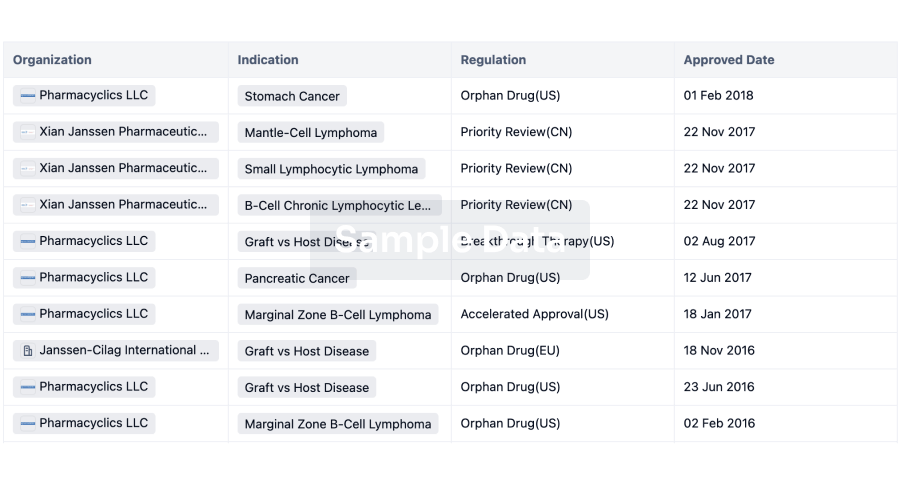
3
Clinical Trials associated with Autologous adipose-derived MSCs(Zhejiang Xingyue Biotechnology) / Unknown statusPhase 2IIT Pilot, single-center, non-comparative clinical trial to assess the safety and preliminary efficacy of the administration of adipose tissue-derived MSCs (MSCs-TA) injected at the edges of the ulcer and assembled three-dimensionally in a fibrin matrix obtained from platelet-rich plasma (MSCs-TA matrix) in patients with chronic wounds non responsive to conventional treatment
Start Date13 Nov 2020 |
Sponsor / Collaborator- |
/ Unknown statusPhase 2IIT Autologous Adipose-derived Mesenchymal Stem Cells in the Treatment of Tendon Disease: a Randomized Controlled Trial
This study was a single-center, randomized, single-blind clinical trial. We plan to include 100 patients who met exclusion criteria of rotator cuff and lateral epicondylosis (tennis elbow) respectively by MRT or ultrasonography. The patients will be randomly divided into two groups. Adipose mesenchymal stem cells will be isolated from adipose tissue, cultured and then transplanted back to the tendon injury site by multiple point injection. 1*10^6 cells as an unit. Patients in the experiment group will be injected into an unit of adipose mesenchymal stem cells (1*10^6/10kg) while the control group received the same dose compound betamethasone injection. Follow up visit for all patients will occur at 1,3,6 and 12 months after the first injection. Clinical quantitative assessment will measure by the visual analogue scale(VAS). The secondary outcomes are the constant-murley score(CMS) and the rating scale of the American shoulder and elbow surgeons(ASES) and the disability of arm shoulder and hand(DASH). The objective evaluation methods is that the examination of MRI or ultrasound were accomplished before the first injection and at 6 and 12 months afterwards.
/ Unknown statusPhase 1/2IIT A Phase I/II Randomized, Controlled, Clinical Trial for Assessment of the Safety and Efficacy of Allogeneic Adipose Mesenchymal Stem Cells in Moderate to Severe Ulcerative Colitis Patients
Ulcerative colitis is a form of inflammatory bowel disease characterized by diffuse inflammation of the colonic mucosa. It affects the rectum and extends proximally along a variable length of the colon. Ulcerative colitis is a chronic condition with a relapsing remitting course. Mesenchymal stem cells (MSCs) are a subset of adult stem cells residing in many tissues, including bone marrow (BM), adipose tissue, umbilical cord blood. Recent experimental findings have shown the ability of MSCs to home to damaged tissues and to produce paracrine factors with anti-inflammatory properties, potentially resulting in reduction of inflammation and functional recovery of the damaged tissues. The purpose of our study is to evaluate safety and efficacy of the intracolonic injection by using a colonoscope of allogeneic adipose MSCs in patients with moderate active ulcerative colitis.
Start Date01 Jul 2018 |
Sponsor / Collaborator- |
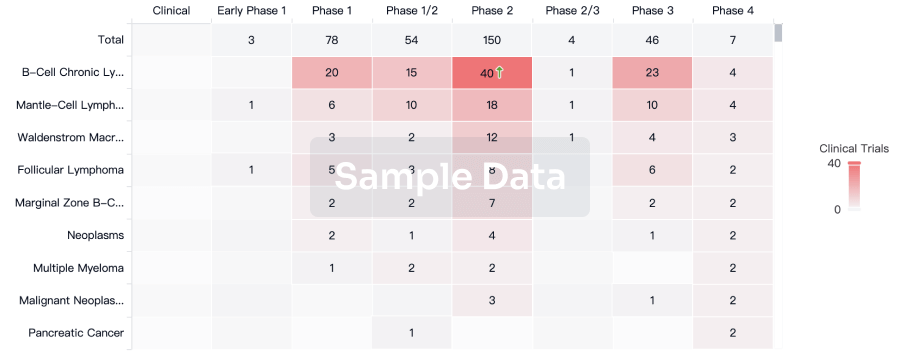
100 Clinical Results associated with Autologous adipose-derived MSCs(Zhejiang Xingyue Biotechnology)
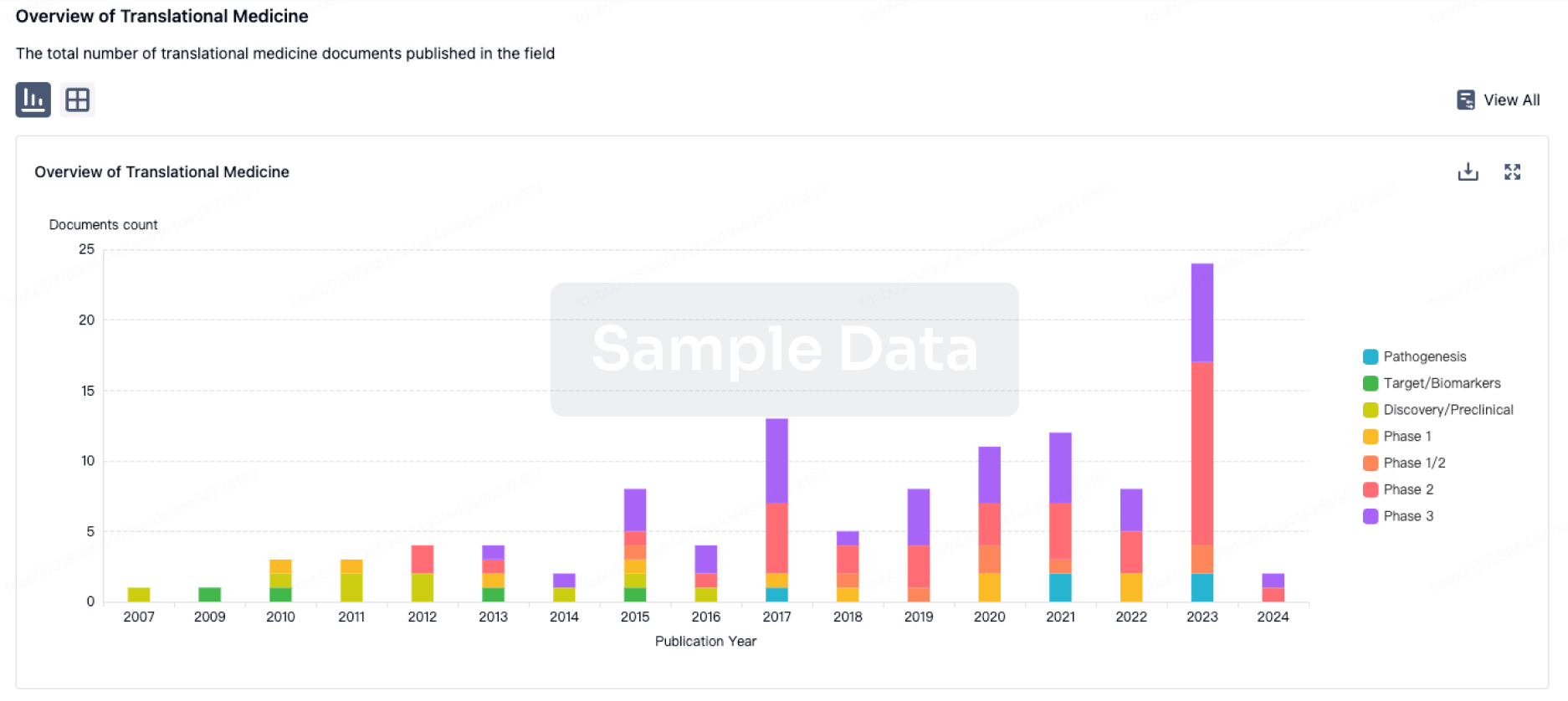
100 Translational Medicine associated with Autologous adipose-derived MSCs(Zhejiang Xingyue Biotechnology)
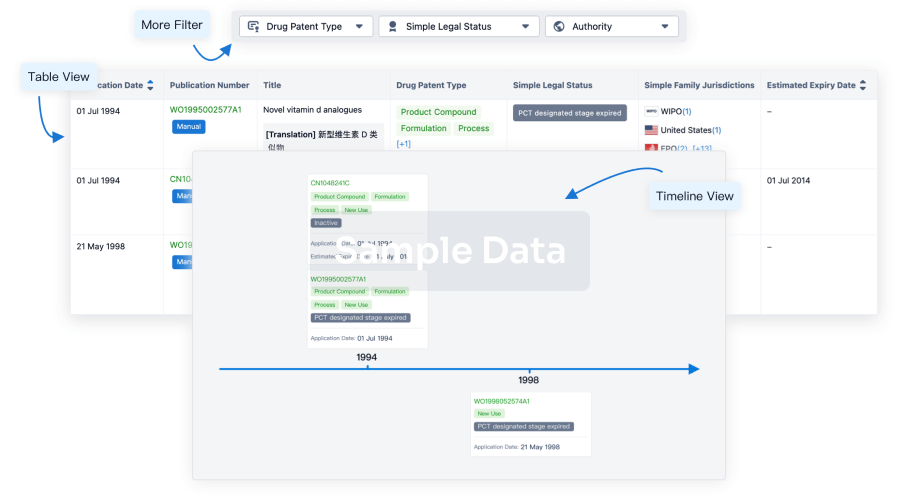
100 Patents (Medical) associated with Autologous adipose-derived MSCs(Zhejiang Xingyue Biotechnology)
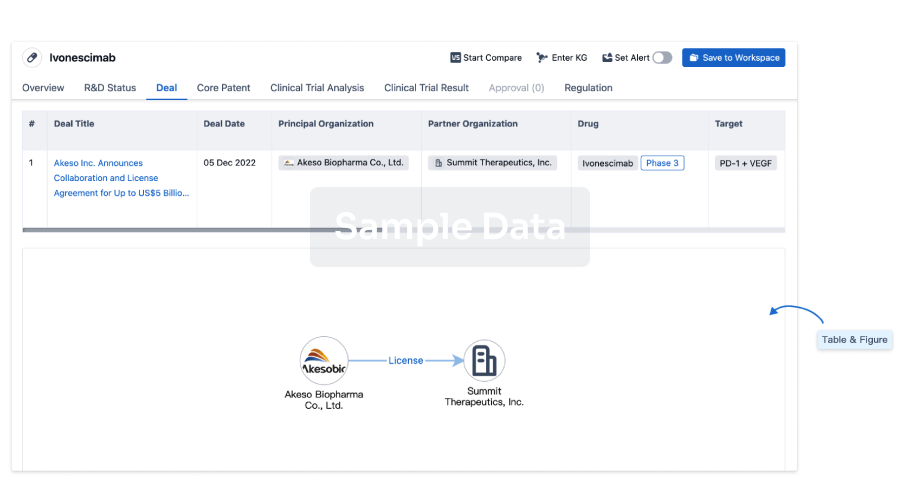
100 Deals associated with Autologous adipose-derived MSCs(Zhejiang Xingyue Biotechnology)