Request Demo
Last update 12 Aug 2025
Human activated protein C(Takeda Pharmaceuticals)
Last update 12 Aug 2025
Overview
Basic Info
Drug Type Blood components |
Synonyms Protein C Concentrate (Human), TAK-662, Ceprotin + [2] |
Target |
Action stimulants |
Mechanism protein C stimulants(Vitamin K-dependent protein C stimulants) |
Active Indication |
Inactive Indication- |
Originator Organization |
Inactive Organization- |
License Organization- |
Drug Highest PhaseApproved |
First Approval Date European Union (16 Jul 2001), |
RegulationOrphan Drug (United States), Orphan Drug (South Korea) |
Login to view timeline
Structure/Sequence
Sequence Code 34059517

Related
7
Clinical Trials associated with Human activated protein C(Takeda Pharmaceuticals)NCT06590974
Special Drug Use Surveillance Study of Ceprotin for Intravenous Injection 1000IU (All-Case Surveillance)
This study is conducted in Japan of Freeze-dried Human Protein C Concentrate (TAK-662) used to treat participants with congenital protein C deficiency.
The main aim of the study is to evaluate for adverse events and effectiveness of congenital protein C deficiency (TAK-662).
During the study, participants with congenital protein C deficiency will be administered with TAK-662 intravenous injection in under routine normal practice. The investigators will evaluate adverse events due to TAK-662 for 12 months. For participants who will be administered in long-term supplementation of TAK-662 after acute treatment or short-term supplementation, the investigator will evaluate for 24 months as a maximum. The study sponsor will not be involved in how the participants are administered but will be recorded what happens during the study.
The main aim of the study is to evaluate for adverse events and effectiveness of congenital protein C deficiency (TAK-662).
During the study, participants with congenital protein C deficiency will be administered with TAK-662 intravenous injection in under routine normal practice. The investigators will evaluate adverse events due to TAK-662 for 12 months. For participants who will be administered in long-term supplementation of TAK-662 after acute treatment or short-term supplementation, the investigator will evaluate for 24 months as a maximum. The study sponsor will not be involved in how the participants are administered but will be recorded what happens during the study.
Start Date06 Sep 2024 |
Sponsor / Collaborator |
NCT04984889
An Open-Label, Single-Dose, Phase 1/2 Study to Evaluate the Pharmacokinetics, Safety, and Tolerability of Human Protein C (TAK-662) for the Treatment of Congenital Protein C Deficiency in Japanese Subjects Followed by an Extension Part
Pharmacokinetic Part:
This study is for Japanese participants with congenital protein C deficiency. The main aim of this study is to check how much TAK-662 stays in their blood over time. This will help the study sponsor (Takeda) to work out the best dose to give patients in the future.
Participants will receive 1 single infusion of TAK-662.
They will stay at the clinic until 3 days after the infusion. Then, participants will return to their clinic 7 days after the infusion to check side effects from the study treatment.
Extension Part:
Participants who will complete the PK part will be given an opportunity to continue TAK-662 administration as 3 different treatment options (on-demand therapy, short-term prophylaxis, and long-term prophylaxis) in the Extension part, until the commercial protein C concentrate is available at each study site or study termination.
This study is for Japanese participants with congenital protein C deficiency. The main aim of this study is to check how much TAK-662 stays in their blood over time. This will help the study sponsor (Takeda) to work out the best dose to give patients in the future.
Participants will receive 1 single infusion of TAK-662.
They will stay at the clinic until 3 days after the infusion. Then, participants will return to their clinic 7 days after the infusion to check side effects from the study treatment.
Extension Part:
Participants who will complete the PK part will be given an opportunity to continue TAK-662 administration as 3 different treatment options (on-demand therapy, short-term prophylaxis, and long-term prophylaxis) in the Extension part, until the commercial protein C concentrate is available at each study site or study termination.
Start Date07 Sep 2021 |
Sponsor / Collaborator |
NCT01705808
Administration of Protein C Concentrates in Adult Critically Ill Septic Patients
Severe sepsis and septic shock are life threatening medical emergencies and are among the most significant challenges in critical care. Case reports and case series suggest that plasma-derived protein C concentrate may improve the outcome of patients with acquired protein C deficiency. Evidence has accumulated on the clinical relevance of the PC pathway in modulating overwhelming inflammation and preventing coagulation derangements, two key mediators of organ damage, and thus of mortality and morbidity, in sepsis. The experience collected through these studies shows that PC is safe, in that it is not associated with bleeding or severe allergic complications,and possibly useful, at least to improve the coagulation abnormalities brought about by sepsis. Unfortunately, however, all we know comes from case series or case reports or an underpowered randomized controlled study. A randomized clinical trial, adequately powered for mortality or clinically relevant outcome, is necessary to confirm PC efficacy.The aim of this study is to demonstrate that Protein C zymogen has clinically relevant implications in terms of reduction of thromboembolic events, 30 days mortality, length of intensive care and hospital stay, time on mechanical ventilation, length of ICU and hospital stay. The study will also confirm that there is no bleeding concern with the use of Protein C concentrates.The study drug will be administered in the Intensive Care Unit for 72 hours and the patients observed till ICU discharge. Telephone followup will be performed at 30 days and at one year.
Start Date01 Sep 2012 |
Sponsor / Collaborator |
100 Clinical Results associated with Human activated protein C(Takeda Pharmaceuticals)
Login to view more data
100 Translational Medicine associated with Human activated protein C(Takeda Pharmaceuticals)
Login to view more data
100 Patents (Medical) associated with Human activated protein C(Takeda Pharmaceuticals)
Login to view more data
100
Literatures (Medical) associated with Human activated protein C(Takeda Pharmaceuticals)01 Jul 2025·JOURNAL OF THROMBOSIS AND HAEMOSTASIS
Real-world use of protein C concentrate for the treatment of patients with protein C deficiency: an international registry
Article
Author: Siffel, Csaba ; Gazda, Hanna T ; Knoebl, Paul ; Turecek, Peter L ; Manco-Johnson, Marilyn ; Brons, Paul ; Wang, Michael
BACKGROUND:
Published reports on the real-world use of protein C concentrate have been limited to case reports or small case series.
OBJECTIVES:
To collect and assess data on the medical diagnoses, treatment regimens, safety outcomes, and treatment outcomes of patients receiving protein C concentrate in routine clinical practice.
METHODS:
This was a prospective, open-label, registry-based study conducted at 26 sites in Europe and the USA. Data were collected from the medical records of patients of all ages who received treatment with protein C concentrate. The primary endpoints were medical diagnoses associated with protein C concentrate treatment, treatment regimens, and the safety of protein C concentrate (incidence of related adverse events [AEs] and all serious AEs [SAEs]).
RESULTS:
Between June 23, 2010, and June 22, 2015, 43 patients, including 25 with severe congenital protein C deficiency (SCPCD) and 18 with severe acquired protein C deficiency, were enrolled in the study, all of whom were included in the analysis. In total, 306 treatment courses of protein C concentrate were documented during the study, for treatment of acute thrombotic episodes, short-term replacement, and long-term prophylaxis. Most patients (76%) with SCPCD were receiving long-term prophylaxis. Overall, 124 AEs were reported in 27 patients (63%); 17 patients experienced SAEs. Only 2 AEs (SAEs of abdominal pain and lower extremity pain) in 1 patient were considered possibly related to protein C concentrate.
CONCLUSION:
The results of this study provide insights into the real-world use of protein C concentrate in clinical practice in Europe and the USA.
01 May 2025·BMJ Case Reports
Compound heterozygous congenital protein C deficiency: a challenging management with recurrent purpura fulminans treated with protein C concentrations
Article
Author: Brandao, Leonardo R ; Sosothikul, Darintr ; Srichumpuang, Chonlatis ; Kor-Anantakul, Phawin
A female newborn was transferred to the hospital due to progressive necrotic skin lesions. She was born full-term with an average birth weight and no prenatal or perinatal problems. She developed the first purplish skin lesion on her left buttock since postnatal day 1. She was initially treated with antibiotics under the impression of early-onset neonatal sepsis; however, her lesions worsened and rapidly progressed. The protein C chromogenic activity was remarkably decreased at below 10%, similar to a protein C antigen level below 1%, highly suggestive of severe congenital protein C deficiency. Molecular analysis revealed compound heterozygous likely pathogenic variants in the protein C gene. After the diagnosis, fresh frozen plasma (FFP) transfusions every 8 hours until the patient was stabilized and therapeutic low-molecular-weight heparin was started. She developed complications related to FFP administration, such as acute kidney injury, hypertension and proteinuria, during the interim period, awaiting the initiation of protein C concentrate replacement therapy.
01 Mar 2025·Research and Practice in Thrombosis and Haemostasis
Evaluation of pharmacokinetics of intravenous protein C concentrate in protein C deficiency: implications for treatment initiation and maintenance
Article
Author: Sorribes, Inmaculada C ; Li, Zhaoyang ; Taylor, Adekemi ; Schneider, Jennifer
Background:
Dosing of intravenous protein C concentrate (Ceprotin) in patients with protein C deficiency is guided by pharmacokinetics (PK) of plasma protein C activity.
Objectives:
This study aimed to characterize PK of intravenous Ceprotin in patients with severe congenital protein C deficiency (SCPCD) and severe acquired protein C deficiency (SAPCD).
Methods:
An exploratory analysis was conducted to assess effects of demographic and disease-related factors on PK of Ceprotin (n = 35 patients with SCPCD or SAPCD), followed by a population PK analysis on data from 4 prospective clinical trials of Ceprotin in SCPCD or SAPCD (n = 58). Model-based simulations were conducted across 3-stage or 1-stage dosing scenarios based on label-recommended doses in acute or maintenance settings.
Results:
Age, body weight, and symptomatic state of disease appeared to influence PK of Ceprotin, including endogenous production of (active) protein C. Model-based simulations predicted that after the first doses, 15% to 76% of patients in the 3-stage dosing scenarios (initial dose: 60-120 IU/kg) and 15% of patients in the 1-stage dosing scenario (60 IU/kg every 12 hours) would attain the recommended target C max of >100 IU/dL. At steady state, ≥86% of patients were predicted to attain the recommended maintenance C trough of >25 IU/dL. The steady-state protein C concentration was driven by the maintenance dose, regardless of the level of initial loading doses.
Conclusion:
Age, body weight, and symptomatic disease state should be considered in dose optimization. Model-based simulations support the use of various combined loading and maintenance regimens to quickly and effectively achieve target protein C plasma levels.
1
News (Medical) associated with Human activated protein C(Takeda Pharmaceuticals)11 Nov 2022
Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 7-10 November 2022
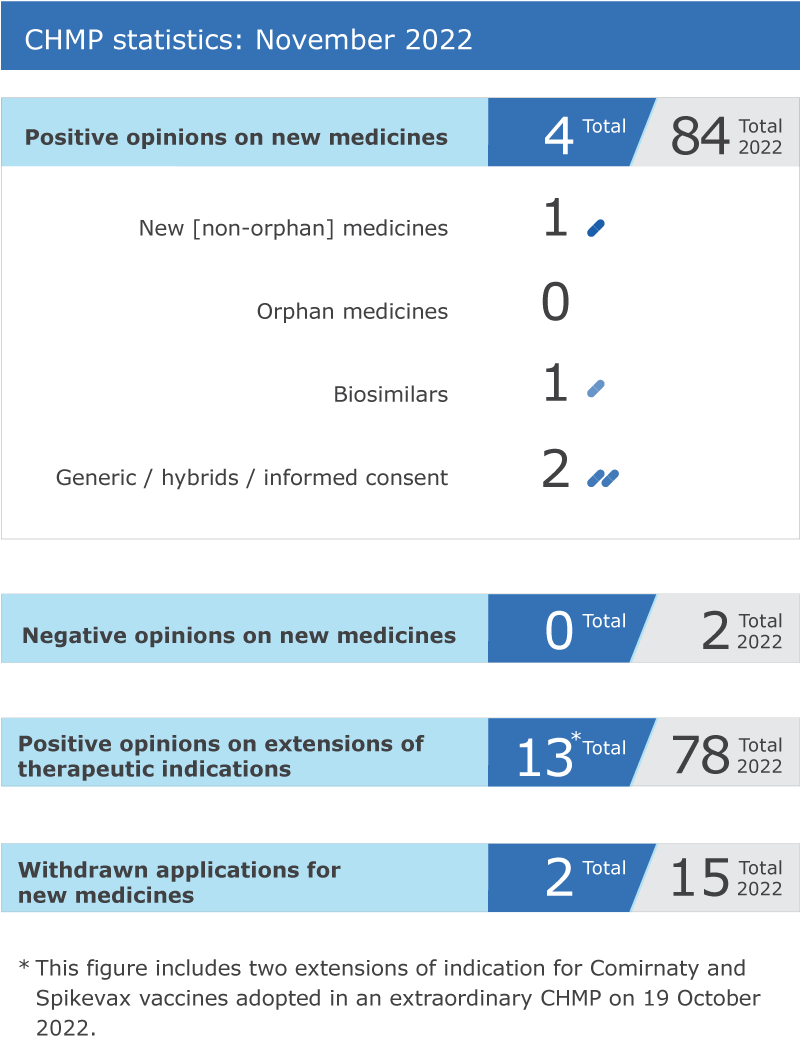
Four new medicines recommended for approval
EMA’s human medicines committee (
CHMP
) recommended four medicines for approval at its November 2022 meeting.
The
CHMP
recommended authorising the COVID-19 vaccine
VidPrevtynBeta
(COVID-19 vaccine (recombinant, adjuvanted)) as a booster in adults previously vaccinated with an mRNA or adenoviral vector COVID-19 vaccine. It is the seventh vaccine recommended in the European Union (EU) for protecting against COVID-19 and, together with the vaccines already authorised, will support vaccination campaigns in EU Member States during the pandemic. See more information in the news announcement in the grid below.
The committee adopted a positive opinion for a
biosimilar medicine
,
Kauliv
(teriparatide), for the treatment of osteoporosis, a health condition that weakens bones, making them fragile and more likely to break.
A
generic medicine
,
Pirfenidone Viatris
(pirfenidone), received a positive opinion for the treatment of idiopathic pulmonary fibrosis, a chronic and progressive condition in which the lungs become scarred and breathing becomes increasingly difficult.
The
CHMP
adopted a positive opinion for a
generic medicine
,
Sugammadex Amomed
(sugammadex), intended for the reversal of neuromuscular blockade induced by rocuronium in adults and children or vecuronium in adults. Rocuronium and vecuronium are muscle relaxants used during some types of surgeries. Sugammadex is used to speed up the recovery from the effects of the muscle relaxant.
Recommendations on extensions of therapeutic indication for 11 medicines
The committee recommended 11 extensions of
indication
for medicines that are already authorised in the EU:
Ceprotin
,
Comirnaty
,
DuoPlavin
,
Dupixent
,
Enhertu
,
Eylea
,
Imfinzi
,
Iscover
,
Lynparza
,
Plavix
and
Xofluza
.
Withdrawals of applications
Two applications for
marketing authorisation
were withdrawn:
Orepaxam
* for the treatment of pulmonary arterial hypertension, and
Febseltiq
* for the treatment of cholangiocarcinoma (cancer of the bile ducts).
Two applications for extensions of therapeutic
indications
were withdrawn:
Gavreto
for the treatment of certain types of thyroid cancer, and
Ilaris
for the treatment of Schnitzler syndrome, a rare inflammatory disease causing long-term urticaria, recurrent fever, bone and joint pain, and swollen lymph nodes.
Question-and-answer documents on the withdrawals are available in the grid below.
COVID-19 update
The committee recommended extending the use of COVID-19 vaccine
Comirnaty
targeting the original strain and Omicron subvariants BA.4 and BA.5 in children between 5 to 11 years of age.
An overview of all the COVID-19 vaccines
authorised in the EU is available on EMA’s website.
Safety update
The
CHMP
endorsed the measures recommended by the
Pharmacovigilance Risk Assessment Committee
(
PRAC
) to minimise the risk of serious side effects with
Janus kinase (JAK) inhibitors
used to treat several chronic inflammatory disorders. These side effects include cardiovascular conditions, blood clots, cancer and serious infections. This recommendation is the outcome of an article 20
referral
procedure, which is triggered for medicines that have been authorised via the
centralised procedure
in case ofquality, safety or
efficacy
issues. A public health communication on this
referral
is available in the grid below.
Agenda and minutes
The agenda of the November 2022
CHMP
meeting is published on EMA's website. Minutes of the October 2022
CHMP
meeting will be published in the coming weeks.
CHMP statistics
Key figures from the November 2022
CHMP
meeting are represented in the graphic below.
*This product was designated as an
orphan medicine
during its development.
Orphan designations
are reviewed by EMA's
Committee for Orphan Medicinal Products
(
COMP
) at the time of approval to determine whether the information available to date allows maintaining the medicine’s orphan status and granting the medicine ten years of
market exclusivity
.
Positive recommendation on new medicines
Name of medicine
VidPrevtyn Beta
International non-proprietary name
(INN)
COVID-19 vaccine (recombinant, adjuvanted)
Marketing-authorisation applicant
Sanofi Pasteur
Therapeutic
indication
VidPrevtyn Beta is indicated as a booster for active immunisation to prevent COVID-19 in adults who have previously received a mRNA or adenoviral vector COVID-19 vaccine
More information
VidPrevtyn Beta: Pending EC decision
News announcement:
EMA recommends approval of VidPrevtyn Beta as a COVID 19 booster vaccine
Positive recommendation on new biosimilar medicine
Name of medicine
Kauliv
INN
teriparatide
Marketing-authorisation applicant
Strides Pharma Cyprus
Therapeutic
indication
Treatment of osteoporosis
More information
Kauliv: Pending EC decision
Positive recommendations on new generic medicines
Name of medicine
Pirfenidone Viatris
INN
pirfenidone
Marketing-authorisation applicant
Viatris Limited
Therapeutic
indication
Treatment of idiopathic pulmonary fibrosis
More information
Pirfenidone Viatris: Pending EC decision
Name of medicine
Sugammadex Amomed
INN
sugammadex
Marketing-authorisation applicant
AOP Orphan Pharmaceuticals GmbH
Therapeutic
indication
Reversal of neuromuscular blockade induced by rocuronium or vecuronium
More information
Sugammadex Amomed: Pending EC decision
Positive recommendations on extensions of indications
Name of medicine
Ceprotin
INN
human protein C
Marketing-authorisation holder
Takeda Manufacturing Austria AG
More information
Ceprotin: Pending EC decision
Name of medicine
Comirnaty
INN
tozinameran
Marketing-authorisation holder
BioNTech Manufacturing GmbH
More information
Comirnaty: Pending EC decision
Name of medicine
DuoPlavin
INN
clopidogrel / acetylsalicylic acid
Marketing-authorisationholder
Sanofi-aventis groupe
More information
DuoPlavin: Pending EC decision
Name of medicine
Dupixent
INN
dupilumab
Marketing-authorisation holder
Sanofi-aventis groupe
More information
Dupixent: Pending EC decision
Name of medicine
Enhertu
INN
trastuzumab deruxtecan
Marketing-authorisation holder
Daiichi Sankyo Europe GmbH
More information
Enhertu: Pending EC decision
Name of medicine
Eylea
INN
aflibercept
Marketing-authorisation holder
Bayer AG
More information
Eylea: Pending EC decision
Name of medicine
Imfinzi
INN
durvalumab
Marketing-authorisation holder
AstraZeneca AB
More information
Imfinzi: Pending EC decision
Name of medicine
Iscover
INN
clopidogrel
Marketing-authorisationholder
Sanofi-aventis groupe
More information
Iscover: Pending EC decision
Name of medicine
Lynparza
INN
olaparib
Marketing-authorisationholder
AstraZeneca AB
More information
Lynparza: Pending EC decision
Name of medicine
Plavix
INN
clopidogrel
Marketing-authorisationholder
Sanofi-aventis groupe
More information
Plavix: Pending EC decision
Name of medicine
Xofluza
INN
baloxavir marboxil
Marketing-authorisationholder
Roche Registration GmbH
More information
Xofluza: Pending EC decision
Withdrawal of initial marketing authorisation application
Name of medicine
Febseltiq
INN
infigratinib
Marketing-authorisation applicant
Helsinn Birex Pharmaceuticals Limited
More information
Febseltiq: Withdrawn application
Name of medicine
Orepaxam
INN
treprostinil diolamine
Marketing-authorisationapplicant
Ferrer Internacional S.A.
More information
Orepaxam: Withdrawn application
Withdrawals of post-authorisation marketing authorisation applications
Name of medicine
Gavreto
INN
pralsetinib
More information
Gavreto: Withdrawn application
Name of medicine
Ilaris
INN
canakinumab
More information
Ilaris: Withdrawn application
Conclusion of referral
Name of medicine
Janus kinase (JAK) inhibitors
More information
Janus Kinase inhibitors (JAKi)
Other updates
List item
Scientific advice and protocol assistance adopted during the CHMP meeting 7-10 November 2022
(PDF/246.92 KB)
(new)
Adopted
First published: 11/11/2022
EMA/CHMP/SAWP/872304/2022
List item
Start of Union reviews adopted during the CHMP meeting of 7-10 November 2022
(PDF/106.5 KB)
(new)
Adopted
First published: 11/11/2022
EMA/846051/2022
VaccineDrug ApprovalOrphan Drug
100 Deals associated with Human activated protein C(Takeda Pharmaceuticals)
Login to view more data
R&D Status
10 top approved records. to view more data
Login
| Indication | Country/Location | Organization | Date |
|---|---|---|---|
| Purpura Fulminans | Japan | 26 Mar 2024 | |
| Venous Thromboembolism | Japan | 26 Mar 2024 | |
| Protein C Deficiency | European Union | 16 Jul 2001 | |
| Protein C Deficiency | Iceland | 16 Jul 2001 | |
| Protein C Deficiency | Liechtenstein | 16 Jul 2001 | |
| Protein C Deficiency | Norway | 16 Jul 2001 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
No Data | |||||||
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or

Deal
Boost your decision using our deal data.
login
or

Core Patent
Boost your research with our Core Patent data.
login
or

Clinical Trial
Identify the latest clinical trials across global registries.
login
or

Approval
Accelerate your research with the latest regulatory approval information.
login
or

Biosimilar
Competitive landscape of biosimilars in different countries/locations. Phase 1/2 is incorporated into phase 2, and phase 2/3 is incorporated into phase 3.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free

