SHANGHAI, Aug. 18, 2022 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, announces that a case report, titled "
Long Term Complete Response of Advanced Hepatocellular Carcinoma to Glypican-3 Specific Chimeric Antigen Receptor T-Cells plus Sorafenib, A case report", has been published in Frontiers in Immunology (https://www.frontiersin.org/articles/10.3389/fimmu.2022.963031/full).
Continue Reading
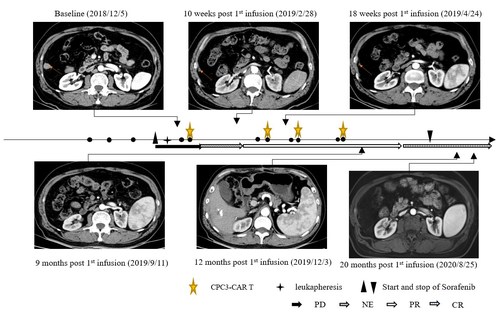
Changes of the No.3 target lesion
Hepatocellular carcinoma (HCC) is the most common histologic subtype of primary liver cancer, which is the sixth most common cancer type worldwide. Clinical efficacies of existing therapies for unresectable HCC are still unsatisfactory. CAR T-cell therapy has been approved for a variety of hematological tumors, but there are still great challenges for CAR T-cell therapies to treat solid tumors. We firstly reported GPC3 as a reasonable target for CAR T-cell therapy and thereafter advanced it into clinic.[1,2] In order to further enhance the efficacy of GPC3 CAR T cells, we proposed a new strategy by combining the GPC3 CAR T cells with sorafenib for the treatment of hepatocellular carcinoma[3]. To further validate this strategy in clinical setting, we conducted an investigator-initiated clinical trial at the First Affiliated Hospital of Wenzhou Medical University.
The published case reported a patient with advanced HCC who achieved a complete response (CR) and a long survival period after the combination therapy of CAR-GPC3 T-cell plus sorafenib.
The case showed a 60-year-old Asian male patient with hepatitis B virus (HBV)-related HCC who underwent surgery in May 2018. In August 2018, the recurrence of liver cancer and pulmonary metastasis occurred after the operation, and then he received transarterial chemoembolization (TACE) to treat liver lesions and interventional ablation to treat pulmonary metastases. Two months later, he progressed and was enrolled into the clinical trial. After the enrollment, the patient underwent leukapheresis for CAR-GPC3 T-cell manufacturing. Seven days after leukapheresis, the patient started to receive 400 mg of sorafenib twice daily. The patient received 4 cycles of CAR-GPC3 T cells (CT011) treatment and each cycle was divided into two infusions. Prior to each cycle of CT011 treatment, lymphodepletion was performed. A total of 4×109 CAR-GPC3 T cells were infused.
The CT011 plus sorafenib combination therapy was well tolerated. This patient obtained partial responses (PR) from the 3rd month and achieved CR in the 12th month after the first cycle of CT011 infusion. The tumor had no progression for more than 36 months and maintained the CR status for more than 24 months after the first infusion.
To the best of our knowledge, this is the first reported case with a CR after the combination therapy of CAR T cells with tyrosine kinase inhibitors. The clinical outcome demonstrated that the combination therapy of GPC3 CAR T-cell and Sorafenib may be a new promising approach for GPC3+ advanced HCC patients.
Dr. Zonghai Li, Chairman of the Board, Chief Executive Officer, and Chief Scientific Officer of CARsgen Therapeutics Holdings Limited, commented that, "There is great expectation for CAR T cells to provide curative potential in treating solid tumors. When enrolled into the clinical trial, the patient in this reported case had undergone local therapies such as TACE and interventional ablation but had not received systemic therapies such as anti-angiogenesis inhibitors. Based on the finding of our earlier preclinical research, we adopted the combination therapy of sorafenib and CT011 as treatment regimens. It was very encouraging to see that the patient achieved a complete response and a long survival period without recurrence for more than two years. While directly indicating that GPC3 CAR T may be used for early-line treatment of HCC, this case report also provides new evidence supporting the adoption of CAR T cells in the early-line treatment of other solid tumors."
About CT011
CT011 is an autologous CAR T-cell product candidate with proof-of-concept clinical data for the treatment of hepatocellular carcinoma (HCC) and has the potential to be the first-in-class globally. Dr. Zonghai Li — Founder, Chairman of the Board, Chief Executive Officer and Chief Scientific Officer of CARsgen Therapeutics — led the world's first successful effort in identifying, validating, and reporting GPC3 as a tumor-associated target for the development of CAR T-cell therapies to treat HCC. CARsgen has completed enrollment of a Phase I trial in China.
About CARsgen Therapeutics Holdings Limited
CARsgen is a biopharmaceutical company with operations in China and the U.S. and is focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors. The Company has built an integrated cell therapy platform with in-house capabilities that span target discovery, antibody development, clinical trials, and commercial-scale manufacturing. CARsgen has internally developed novel technologies and a product pipeline with global rights to address major challenges of CAR T-cell therapies, such as improving the safety profile, enhancing the efficacy in treating solid tumors and reducing treatment costs. The Company's vision is to become a global biopharmaceutical leader that brings innovative and differentiated cell therapies to cancer patients worldwide and makes cancer curable.