Request Demo
Last update 22 Nov 2025
SRSD-101
Last update 22 Nov 2025
Overview
Basic Info
Drug Type siRNA |
Synonyms SRS 001, SRS001, SRSD 101 + [1] |
Target |
Action inhibitors |
Mechanism PCSK9 inhibitors(Proprotein convertase subtilisin kexin type 9 inhibitors) |
Therapeutic Areas |
Active Indication |
Inactive Indication- |
Originator Organization |
Active Organization |
Inactive Organization- |
License Organization- |
Drug Highest PhasePhase 1 |
First Approval Date- |
Regulation- |
Login to view timeline
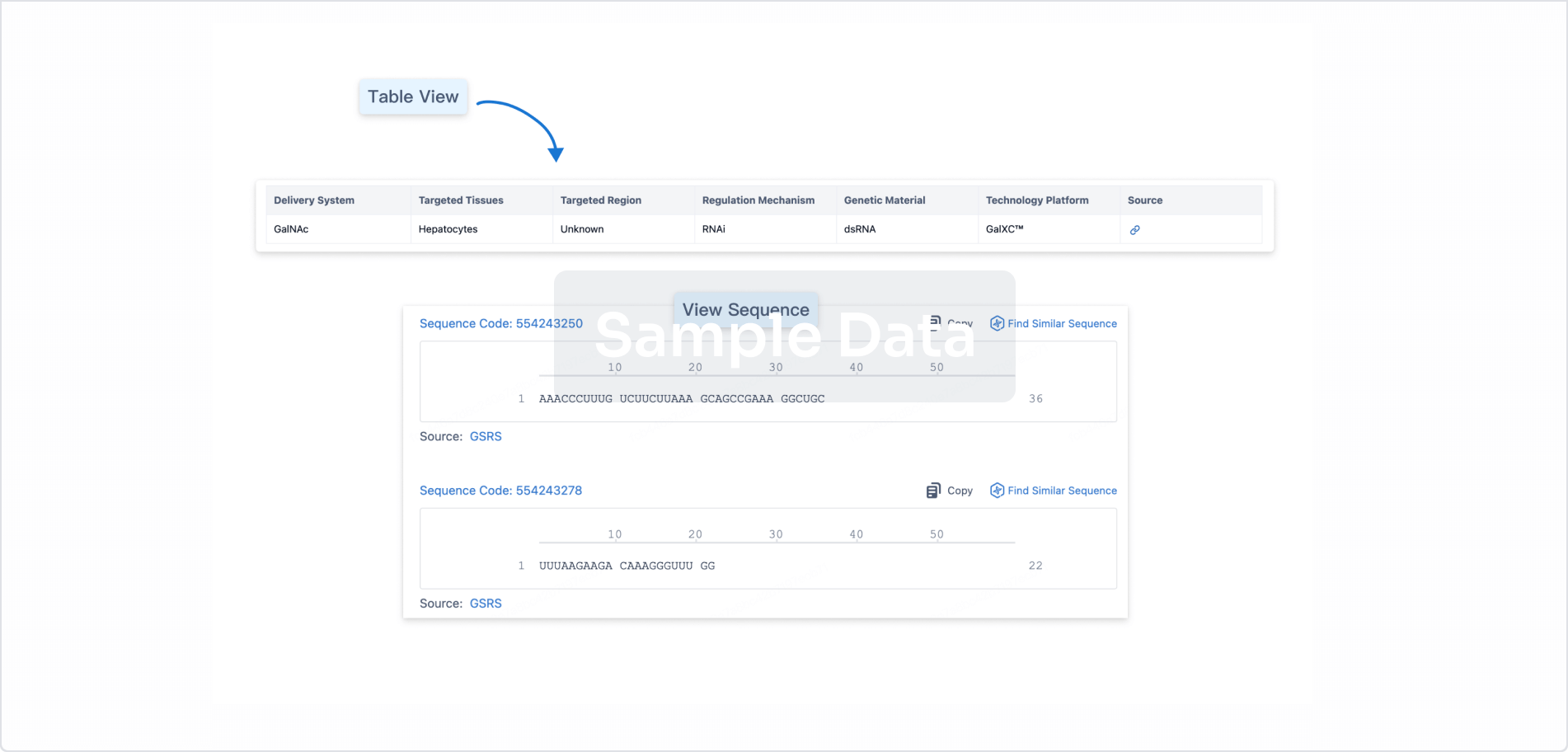
Structure/Sequence
Boost your research with our RNA technology data.
login
or
Related
1
Clinical Trials associated with SRSD-101CTR20233758
一项在低密度脂蛋白胆固醇正常或升高的中国受试者中评价SRSD101 注射液单剂量皮下给药剂量递增安全性、耐受性、药代动力学和药效学特征的随机、双盲、安慰剂对照的I期临床研究
[Translation] A randomized, double-blind, placebo-controlled Phase I clinical study to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of SRSD101 injection administered subcutaneously in Chinese subjects with normal or elevated low-density lipoprotein cholesterol
主要目的:评价单剂量皮下给药后SRSD101 在LDL C 正常或升高的中国受试者中的安全性和耐受性。
次要目的:评价单剂量皮下给药后SRSD101 及其血浆代谢产物(如适用)在LDL C 正常或升高的中国受试者中的药代动力学(PKPK)特征;评价单剂量皮下给药后SRSD101 在LDL C 正常或升高的中国受试者中的药效学(PDPD)特征;评价单剂量皮下给药后SRSD101 在LDL C 正常或升高的中国受试者中的免疫原性(ADAADA)特征。
[Translation]
Primary objective: To evaluate the safety and tolerability of SRSD101 in Chinese subjects with normal or elevated LDL C after a single subcutaneous dose.
Secondary objectives: To evaluate the pharmacokinetic (PKPK) characteristics of SRSD101 and its plasma metabolites (if applicable) in Chinese subjects with normal or elevated LDL C after a single subcutaneous dose; to evaluate the pharmacodynamic (PDPD) characteristics of SRSD101 in Chinese subjects with normal or elevated LDL C after a single subcutaneous dose; to evaluate the immunogenicity (ADAADA) characteristics of SRSD101 in Chinese subjects with normal or elevated LDL C after a single subcutaneous dose.
Start Date30 Nov 2023 |
Sponsor / Collaborator |
100 Clinical Results associated with SRSD-101
Login to view more data
100 Translational Medicine associated with SRSD-101
Login to view more data
100 Patents (Medical) associated with SRSD-101
Login to view more data
4
News (Medical) associated with SRSD-10120 May 2025
-Collaboration brings together complementary capabilities to co-develop and co-commercialize SRSD107, a next generation, long-acting Factor XI (FXI)
small interfering RNA (siRNA) for the treatment of thromboembolic disorders-
-SRSD107 demonstrated peak reductions in FXI activity >93% and increases in activated partial thromboplastin time (aPTT) >2x with maintained efficacy up to 6 months post-dosing in a Phase 1 clinical trial-
-Under the agreement, CRISPR Therapeutics will make an upfront payment of $25 million in cash and $70 million in equity to Sirius Therapeutics; CRISPR Therapeutics also has rights to exclusively license up to two additional siRNA programs-
-Expands CRISPR’s therapeutic toolkit to develop a broader range of transformative gene-based medicines in addition to the gene-editing programs in the clinic-
ZUG, Switzerland and BOSTON, MA, USA and SAN DIEGO, CA, USA and SHANGHAI, China I May 19, 2025 I
CRISPR Therapeutics (NASDAQ: CRSP), a biopharmaceutical company focused on creating transformative gene-based medicines for serious diseases, and Sirius Therapeutics, a clinical stage biotech company developing innovative small interfering RNA (siRNA) therapies for global markets, today announced a strategic partnership to develop and commercialize siRNA therapies.
“We are excited to partner with Sirius, and broaden our cardiovascular medicine portfolio, on the heels of promising top-line data that we recently shared for CTX310, which targets ANGPTL3,” said Samarth Kulkarni, Ph.D., Chairman and Chief Executive Officer of CRISPR Therapeutics. “Coagulation Factor XI represents an innovative and highly compelling target for treating thrombotic diseases that affect millions worldwide. SRSD107, which targets Factor XI, has the potential to be a best-in-class therapy, offering infrequent dosing and improved patient outcomes. Sirius’ siRNA platform complements our existing capabilities and expands our therapeutic toolkit, enabling us to develop a broader range of transformative gene-based medicines.”
“We are pleased to collaborate with CRISPR Therapeutics, a recognized leader in the development of gene-based medicines,” said Qunsheng Ji, MD, Ph.D. Chief Executive Officer of Sirius Therapeutics. “Thrombotic diseases represent a significant unmet need, and our promising Phase 1 data highlights the potential of SRSD107 as a best-in-class Factor XI-targeted therapy. Sirius is committed to addressing the needs of these patients, as we work with CRISPR Therapeutics to advance novel siRNA therapies globally.”
“There is a large population of patients who are at risk for potentially life-threatening thromboembolic events due to underlying co-morbid diseases such as malignancy, cardiovascular disease, and hyper-coagulability. A significant percentage of these patients are inadequately treated due to concerns for bleeding risk, or challenges with compliance,” said Christian T. Ruff, M.D., M.P.H., senior investigator of TIMI Group, director General Cardiology, Brigham and Women’s Hospital, and associate professor, Harvard Medical School. “SRSD107 offers the potential for a therapy with lower bleeding risk, infrequent dosing for better compliance, without concerns for renal clearance or drug interactions, and reversibility to further mitigate bleeding risks that could be differentiated from currently available therapies and other Factor XI modalities.”
SRSD107 is a next generation, long-acting siRNA designed to selectively inhibit Factor XI (FXI), a key driver of pathological thrombosis with minimal impact on normal hemostasis. By targeting FXI, SRSD107 aims to reduce thrombotic events while minimizing the risk of bleeding – representing a differentiated approach compared to Factor Xa inhibitors. In addition, SRSD107 may offer the potential for reversibility not observed with other anti-Factor XI modalities. The addressable population includes patients with atrial fibrillation, venous thromboembolism (VTE), cancer-associated thrombosis, chronic Coronary Artery Disease (CAD), chronic Peripheral Vascular Disease (PVD), end-stage renal disease requiring hemodialysis, and patients undergoing major orthopedic surgery, where bleeding risk limits existing therapies.
The clinical program for SRSD107 includes two promising Phase 1 clinical trials, where single doses of SRSD107 were found to be safe and well tolerated. In addition, SRSD107 demonstrated robust pharmacodynamic effects, including reductions of over 93% in FXI levels and FXI activity (FXIa), along with more than a twofold increase in activated partial thromboplastin time (aPTT) relative to baseline. These effects were sustained, with responses maintained for up to 6 months post-dosing. SRSD107 has the potential to be a best-in-class FXI inhibitor, showing deep reductions in FXI via semi-annual subcutaneous injection. Results from the Phase 1 trials were presented at both the 2025 Annual Scientific Sessions of the American College of Cardiology and the 2024 Annual Meeting of the American Society of Hematology.
Figure 1. SRSD107 Phase 1 Clinical Results: Sustained, dose-dependent pharmacodynamic response to therapy
A Phase 2 clinical trial of SRSD107 is being initiated to evaluate its safety and efficacy for the prevention of VTE in patients undergoing total knee arthroplasty. The trial aims to confirm the anticoagulant benefits of SRSD107 and to inform dose selection for future pivotal trials.
Collaboration Details
Under the terms of the agreement, CRISPR Therapeutics will make an upfront payment of $25 million in cash and $70 million in equity to Sirius Therapeutics. The companies will jointly develop SRSD107 under a 50-50 cost and profit-sharing structure. CRISPR Therapeutics will lead commercialization in the U.S., while Sirius will be responsible for commercialization in Greater China.
Additionally, CRISPR Therapeutics will have the option to nominate up to two siRNA targets for research and development. For each target, CRISPR Therapeutics will fund research and retain opt-in rights to lead clinical development and commercialization. Sirius will be eligible to receive milestone payments, as well as tiered royalties ranging from high single to low-double digits.
About CRISPR Therapeutics
Since its inception over a decade ago, CRISPR Therapeutics has evolved from a research-stage company advancing gene editing programs into a leader that celebrated the historic approval of the first-ever CRISPR-based therapy. The Company has a diverse portfolio of product candidates across a broad range of disease areas including hemoglobinopathies, oncology, regenerative medicine, cardiovascular, autoimmune, and rare diseases. In 2018, CRISPR Therapeutics advanced the first-ever CRISPR/Cas9 gene-edited therapy into the clinic to investigate the treatment of sickle cell disease and transfusion-dependent beta thalassemia. Beginning in late 2023, CASGEVY
®
(exagamglogene autotemcel [exa-cel]) was approved in several countries to treat eligible patients with either of these conditions. The Nobel Prize-winning CRISPR technology has revolutionized biomedical research and represents a powerful, clinically validated approach with the potential to create a new class of potentially transformative medicines. To accelerate and expand its efforts, CRISPR Therapeutics has formed strategic partnerships with leading companies including Vertex Pharmaceuticals. CRISPR Therapeutics AG is headquartered in Zug, Switzerland, with its wholly-owned U.S. subsidiary, CRISPR Therapeutics, Inc., and R&D operations based in Boston, Massachusetts and San Francisco, California. To learn more, visit
www.crisprtx.com
.
CRISPR THERAPEUTICS
®
standard character mark and design logo, CTX310™ and CTX320™ are trademarks and registered trademarks of CRISPR Therapeutics AG. CASGEVY
®
and the CASGEVY logo are registered trademarks of Vertex Pharmaceuticals Incorporated. All other trademarks and registered trademarks are the property of their respective owners.
About Thromboembolic Disorders
Thrombosis, or blood clot formation, is the common underlying mechanism of most cases of myocardial infarction, ischemic stroke, and venous thromboembolism. According to a trial in The Lancet
1
of regional and global mortality rates, thromboembolic disorders are estimated to cause as many as 1 in 4 deaths worldwide.
About SRSD107
SRSD107 is a novel double-stranded small interfering ribonucleic acid (siRNA). SRSD107 specifically targets the human coagulation factor XI (FXI) mRNA and inhibits FXI protein expression, thereby blocking the intrinsic coagulation pathway and promoting anticoagulant/anti-thrombotic effects. SRSD107 has been engineered for the potential to enable twice-a-year dosing.
About Sirius Therapeutics
Sirius is a clinical stage biotech company developing innovative siRNA therapies for global markets. We are dedicated to discovering and developing new treatment options for cardiovascular and cerebrovascular disease and translating siRNA technology into transformative medicine for chronic disease patients. Sirius’s most advanced products are SRSD107 for the treatment of thromboembolic disorders, SRSD216 for the treatment of hyperlipoproteinemia, and SRSD101 for the treatment of dyslipidemia.
Founded in 2021 by a world-class leadership team and investors, Sirius has established an innovation center in the United States and translational medicine center in China. Sirius has raised nearly US$150 million funding to date from OrbiMed, Creacion Ventures, Hankang Capital, Delos Capital, and BioTrack Capital. Learn more at
www.siriusrna.com
References:
1. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380, 2095-1128.
SOURCE:
CRISPR Therapeutics
Clinical ResultPhase 1License out/inDrug ApprovalPhase 2
09 May 2025
SAN DIEGO & SHANGHAI--(BUSINESS WIRE)--Sirius Therapeutics today announced that it has successfully completed nearly $50 million Series B2 financing to advance clinical development of the Company’s novel siRNA therapeutics for cardiometabolic disorders and continued innovation of its next-generation RNA delivery technologies. A renowned corporate venture capital firm led the financing round that was joined by a new investor, BioTrack Capital, and existing investors OrbiMed, Creacion Ventures, and Hankang Capital.
“The successful completion of our Series B2 financing is a strong endorsement of our progress to date and our strategy going forward,” said Dr. Qunsheng Ji, CEO of Sirius Therapeutics. “We are deeply grateful to our new and existing investors for their continued support. The funds from this round will further advance our clinical programs and expand our pipeline, to deliver “transformative siRNA therapeutics for patients with chronic diseases” around the world.”
Dr. Ji noted that the company has three clinical-stage programs and a deep preclinical pipeline. The most advanced compound, SRSD107, is a long-acting, next-generation anticoagulant for thromboembolic disorders poised to begin Phase 2 clinical development in Europe. The company recently applied to the European Medicines Agency (EMA) to initiate a Phase II trial. Sirius has also received approvals from the U.S. FDA and the China NMPA and has begun Phase 1 studies with SRSD216, a novel siRNA therapeutic for hyperlipoproteinemia in patients with atherosclerotic cardiovascular disease. SRSD101, the company’s siRNA therapeutic for dyslipidemia, is undergoing Phase 1 clinical trials in China.
About Sirius Therapeutics
Sirius is developing transformative siRNA therapeutics for patients with chronic diseases globally. Founded in 2021, Sirius established an innovation center in the United States and a translational medicine center in China dedicated to state-of-the-art solutions for the treatment and management of chronic diseases. The company has successfully raised nearly $150 million to date. For more information, please visit www.siriusrna.com.
Phase 1Phase 2INDsiRNA
10 Dec 2024
Data presented at American Society of Hematology annual meeting
SAN DIEGO, CA, USA & SHANGHAI, China I December 09, 2024 I
Sirius Therapeutics today announced promising preliminary data from its Phase 1 first-in-human clinical trial of SRSD107, a next generation siRNA therapeutics under clinical development for the prevention and treatment of thromboembolic disorders, such as myocardial infarction, ischemic stroke, and venous thromboembolism. The trial data were presented during a poster session at the 66th American Society of Hematology (ASH) Annual Meeting and Exposition, held in San Diego, CA.
SRSD107 is designed to inhibit Factor XI (FXI), a protein in the coagulation pathway that has shown potential to reduce thrombosis without significantly increasing the risk of bleeding, a major liability of current drugs.
“In this trial, SRSD107 was safe and well tolerated, with pharmacokinetic parameters consistent with a typical siRNA product,” said Dr. Patrick Yue, Sirius’ Chief Medical Officer. “We are encouraged by the marked, prolonged reduction in FXI antigen and activity, and increase in clotting, or thromboplastin time, that are consistent potent anticoagulation over sustained periods and reduced bleeding risk respectively. The trial provides a strong foundation for Phase 2 clinical studies.”
Dr. Qunsheng Ji, Sirius’ Chief Executive Officer added that “presentation of these findings at ASH 2024 underscores Sirius Therapeutics’ commitment to advancing innovative treatments for thromboembolic disorders.”
The study was a single-site, randomized double-blind, placebo controlled study to evaluate the safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of subcutaneously administered SRSD107 in 40 healthy subjects. Five cohorts, each consisting of eight subjects evaluated eight doses of SRSD107, each receiving either a single SRSD107 dose or placebo. In the trial, SRSD107 was safe and well-tolerated. Significant changes from baseline in PD biomarkers were observed, with maximal reductions in FXI antigen and activity > 90% and an aPTT (thromboplastin time) increase of > 100% (i.e., an aPTT ratio > 2.0) at the highest doses tested. The pharmacodynamic effects were durable, with FXI antigen and activity levels remaining suppressed for more than 16 weeks after dosing.
The study is titled, “A Phase 1, Single Ascending Dose Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Subcutaneously Administered SRSD107 in Healthy Subjects. For more information on the study and our ongoing clinical trials, please visit Sirius Therapeutics’ website (
www.siriusrna.com
) or
here
.
About Thromboembolic Disorders
Thrombosis, or blood clot formation, is the common underlying mechanism of most cases of myocardial infarction, ischemic stroke, and venous thromboembolism. According to a study in
The Lancet
of regional and global mortality rates, thromboembolic disorders are estimated to cause as many as 1 in 4 deaths worldwide.
About SRSD107
SRSD107 is a novel double-stranded small interfering ribonucleic acid (siRNA). Developed by Sirius Therapeutics, SRSD107 specifically targets human coagulation factor XI (FXI) mRNA and inhibits FXI protein expression, thereby blocking the intrinsic coagulation pathway and promoting anticoagulant/anti-thrombotic effects. SRSD107 has been engineered for the potential to enable once or twice-a-year dosing.
About Sirius Therapeutics
Sirius is an innovative, clinical stage biotech company developing next generation siRNA therapy for global markets. We are dedicated to translating siRNA technology into transformative medicine for patients with chronic diseases. Our most advanced products are SRSD107 for the treatment of thromboembolic disorders, and SRSD101 for the treatment of dyslipidemia. Founded in 2021 by a world-class leadership team and investors, Sirius has established an innovative discovery center in the United States and translational medicine center in China. Sirius has raised nearly US$100 million funding to date from OrbiMed, Creacion Ventures, Hankang Capital, Delos Capital, and the leadership team.
SOURCE:
Sirius Therapeutics
Phase 1Clinical ResultASH
100 Deals associated with SRSD-101
Login to view more data
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Primary hypercholesterolemia | IND Approval | China | 15 Nov 2023 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
No Data | |||||||
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or

Deal
Boost your decision using our deal data.
login
or

Core Patent
Boost your research with our Core Patent data.
login
or

Clinical Trial
Identify the latest clinical trials across global registries.
login
or

Approval
Accelerate your research with the latest regulatory approval information.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free
