Request Demo
Last update 08 May 2025
CARTs(University of Pennsylvania)
Last update 08 May 2025
Overview
Basic Info
Drug Type CAR-T |
Synonyms- |
Target- |
Action- |
Mechanism Immunologic cytotoxicity, T lymphocyte replacements |
Therapeutic Areas |
Active Indication |
Inactive Indication- |
Originator Organization |
Active Organization |
Inactive Organization- |
License Organization- |
Drug Highest PhasePreclinical |
First Approval Date- |
Regulation- |
Related
100 Clinical Results associated with CARTs(University of Pennsylvania)
Login to view more data
100 Translational Medicine associated with CARTs(University of Pennsylvania)
Login to view more data
100 Patents (Medical) associated with CARTs(University of Pennsylvania)
Login to view more data
108
Literatures (Medical) associated with CARTs(University of Pennsylvania)01 Mar 2025·Journal of Allergy and Clinical Immunology
Chimeric antigen receptor–modified T-cell therapy: Recent updates and challenges in autoimmune diseases
Review
Author: Caël, Blandine ; Garnache Ottou, Francine ; Bôle-Richard, Elodie ; Aubin, François
01 Dec 2024·International Immunopharmacology
Advancements in the design and function of bispecific CAR-T cells targeting B Cell-Associated tumor antigens
Review
Author: Sima, Helin ; Shao, Wenwei
01 Sep 2024·Cytotherapy
Optimizing lentiviral vector formulation conditions for efficient ex vivo transduction of primary human T cells in chimeric antigen receptor T-cell manufacturing
Article
Author: Kaartinen, Tanja ; Ylä-Herttuala, Seppo ; Käyhty, Piia ; Lesch, Hanna P ; Turkki, Vesa ; Albers-Skirdenko, Vita ; Kailaanmäki, Anssi ; Leinonen, Hanna ; Köylijärvi, Marjut ; Lipponen, Eevi ; Kekarainen, Tuija ; Luostarinen, Annu
29
News (Medical) associated with CARTs(University of Pennsylvania)24 Mar 2025
As a BCMA-targeting CAR T-cell therapy, CARVYKTI addresses a high unmet need in relapsed or refractory multiple myeloma patients. With promising clinical efficacy, durable responses, and growing adoption in advanced treatment lines, CARVYKTI is poised for substantial revenue growth.
LAS VEGAS, March 24, 2025 /PRNewswire/ -- DelveInsight's "
CARVYKTI Market Size, Forecast, and Market Insight Report" highlights the details around CARVYKTI, a BCMA-directed, genetically modified autologous T-cell immunotherapy, which involves reprogramming a patient's T cells with a transgene encoding a CAR that identifies and eliminates cells that express BCMA. The report provides product descriptions, patent details, and competitor products (marketed and emerging therapies) of CARVYKTI. The report also highlights the historical and forecasted sales from 2020 to 2034 segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Janssen's CARVYKTI (ciltacabtagene autoleucel) Overview
CARVYKTI is an autologous T-cell immunotherapy that targets B-cell maturation antigen (BCMA). It works by genetically modifying a patient's T cells with a transgene that encodes a chimeric antigen receptor (CAR). This CAR helps the T cells recognize and destroy cells expressing BCMA, which is primarily found in malignant multiple myeloma B-lineage cells, late-stage B cells, and plasma cells. The CARVYKTI CAR protein includes two single-domain antibodies designed to bind strongly to human BCMA, enhancing T-cell activation, proliferation, and the destruction of target cells upon binding.
Currently, CAR-T cell therapies are only available to UK patients through clinical trials. Janssen recently decided not to proceed with seeking approval for cilta-cel (CARVYKTI) for myeloma patients in the UK through the National Institute for Health and Care Excellence (NICE). This decision means cilta-cel will not be available on the NHS for now, although it remains accessible through clinical trials. Janssen's decision does not impact ongoing trials. The drug is currently being evaluated in Phase III trials: CARTITUDE-6 for frontline multiple myeloma transplant eligible vs ASCT and CARTITUDE-5 for frontline multiple myeloma TNI. In 2024, CARVYKTI generated sales of
USD 963 million across the world.
CARVYKTI Dosage and Administration
CARVYKTI is administered as a single infusion containing a suspension of CAR-positive, viable T cells in one infusion bag. The recommended dosage ranges from 0.5 to 1.0 × 10⁶ CAR-positive viable T cells per kilogram of body weight, with a maximum limit of 1 × 10⁸ CAR-positive viable T cells per infusion.
Learn more about CARVYKTI projected market size for multiple myeloma @
CARVYKTI Market Potential
CAR-T cell immunotherapy is emerging as a revolutionary treatment for multiple myeloma, offering new hope to patients who have exhausted conventional treatment options. Currently, only two CAR-T cell therapies are approved for multiple myeloma:
ABECMA (idecabtagene vicleucel) from Bristol-Myers Squibb and
CARVYKTI (ciltacabtagene autoleucel) from Johnson & Johnson Innovative Medicine. Their approval highlights the potential of CAR-T therapy to transform multiple myeloma treatment.
A key advantage of CAR-T therapies is their "one-and-done" nature, requiring a single administration, unlike bispecific antibodies such as
TECVAYLI and TALVEY, which need ongoing dosing. Present CAR-T therapies use a patient's own T cells (autologous), but research is underway to develop allogeneic CAR-T cells derived from healthy donors. Allogeneic therapies could offer off-the-shelf solutions, enhancing accessibility and lowering costs.
The approval of CAR-T therapies has created new opportunities for companies focused on advanced-stage multiple myeloma (fourth line and beyond). Several companies are progressing with their CAR-T candidates at different stages of development.
The CAR-T cell therapy market for multiple myeloma is expected to grow significantly between 2024 and 2034, driven by its high efficacy and potential for expanded use. Collaboration between pharmaceutical companies and research institutions, along with ongoing innovation, is expected to accelerate market growth. However, challenges such as manufacturing complexity and pricing will need to be addressed to unlock the full potential of CAR-T therapies.
Discover more about the CAR T-cell therapy for multiple myeloma market in detail @
CAR T-cell Therapy for Multiple Myeloma Market Report
Emerging Competitors of CARVYKTI
Some of the CAR-Ts in the multiple myeloma pipeline that will give fierce competition to CARVYKTI include
Anito-cel (Arcellx),
PHE885 (Novartis),
BMS-986393 (Bristol-Myers Squibb),
Zevorcabtagene Autoleucel (CARsgen Therapeutics), and
GLPG5301 (Galapagos), among others
In
December 2024, Arcellx announced encouraging new data from its Phase II pivotal
iMMagine-1 trial for anito-cel in multiple myeloma. The results were presented during an oral session at the 66th American Society of Hematology (ASH) Annual Meeting on Monday, December 9, 2024, at 5:30 p.m. PT. Also at ASH 2024,
BMS shared the first overall survival (OS) and progression-free survival (PFS) results for arlo-cel in an oral presentation. The Phase I trial involved patients with relapsed or refractory multiple myeloma who had previously undergone at least three treatments, including a proteasome inhibitor, an immunomodulatory drug, and anti-CD38 therapy.
Among 79 patients evaluable for efficacy, with a median follow-up of 16.1 months (range: 2.8–25.2), arlo-cel showed an overall response rate (ORR) of 87%, indicating durable responses. Minimal residual disease (MRD) was assessed as an exploratory measure, with 57% (48 of 84) of MRD-evaluable patients included. Of these, 46% (22 of 48) were MRD-negative and achieved a complete response (CR) or stringent CR (sCR). Across all treated patients, 27% (23 of 84) were MRD-negative and achieved a CR. Median PFS was 18.3 months (95% CI: 11.8–21.9), while the median OS had not yet been reached.
To know more about the number of competing drugs in development, visit @
CARVYKTI Market Positioning Compared to Other Drugs
Key Milestones of CARVYKTI
In
April 2024, Johnson & Johnson announced that the US FDA approved CARVYKTI (ciltacabtagene autoleucel; cilta-cel) for the treatment of adult patients with RRMM who have received at least one prior line of therapy, including a proteasome inhibitor and an immunomodulatory agent, and are refractory to lenalidomide.
In
March 2024, Johnson & Johnson announced that the US FDA Oncologic Drugs Advisory Committee (ODAC) recommends CARVYKTI for the treatment of adult patients with RRMM who have received at least one prior line of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent (IMiD) and who are refractory to lenalidomide.
In
February 2024, Johnson & Johnson announced that the Committee for Medicinal Products for Human Use (CHMP) of the EMA recommended the approval of a Type II variation for CARVYKTI for the earlier treatment of RRMM.
In
September 2022, Legend Biotech announced that Japan's Ministry of Health, Labour and Welfare (MHLW) had approved CARVYKTI (ciltacabtagene autoleucel) for the treatment of adults with relapsed or refractory multiple myeloma, limited to cases meeting both of the following conditions including patients having no history of CAR-positive T-cell infusion therapy targeting BCMA and patients who have received three or more lines of therapies, including an immunomodulatory agent, a proteasome inhibitor and an anti-CD38 monoclonal antibody, and in whom multiple myeloma has not responded to or has relapsed following the most recent therapy.
In
May 2022, Janssen announced that the European Commission (EC) granted conditional marketing authorization for CARVYKTI for the treatment of adults with relapsed and refractory multiple myeloma (RRMM) who have received at least three prior therapies, including an immunomodulatory agent (IMiD), a proteasome inhibitor (PI) and an anti-CD38 antibody, and have demonstrated disease progression on the last therapy.
In
February 2022, Janssen announced that the US FDA had approved CARVYKTI (ciltacabtagene autoleucel; cilta-cel) for the treatment of adults with relapsed or refractory multiple myeloma (RRMM) after four or more prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.
In
August 2020, Legend Biotech announced that the China Center for Drug Evaluation, National Medical Products Administration (CDE, NMPA) had recommended BTD for ciltacabtagene autoleucel (cilta-cel; LCAR-B38M CAR-T cells) for the treatment of adults with relapsed or refractory multiple myeloma.
In
December 2019, Janssen announced receipt of a BTD from the US FDA for cilta-cel.
In
April 2019, the EMA granted a PRIME (PRIority MEdicines) designation for the company's investigational BCMA CAR-T therapy.
In
February 2019, the US FDA granted ODD for cilta-cel (JNJ-4528), and in February 2020, the European Commission also granted orphan designation for this drug.
In
December 2017, Janssen entered a worldwide collaboration and license agreement with Legend Biotech to jointly develop and commercialize LCAR-B38M in multiple myeloma
Discover how CARVYKTI is shaping the multiple myeloma treatment landscape @
CARVYKTI CAR-T
CARVYKTI Market Dynamics
The market for CARVYKTI is driven by the
increasing prevalence of multiple myeloma and the
growing demand for innovative therapies that can address the limitations of existing treatments, such as proteasome inhibitors and immunomodulatory drugs. The competitive landscape includes other BCMA-targeting therapies, such as
Bristol Myers Squibb's ABECMA (idecabtagene vicleucel), which was the first BCMA-directed CAR-T therapy approved for multiple myeloma.
CARVYKTI's strong clinical efficacy, coupled with its potential for long-term remission, gives it a competitive edge, but challenges related to
manufacturing scalability, logistical complexities, and high treatment costs could impact its market penetration and patient accessibility.
Strategic partnerships and ongoing clinical development will play a key role in expanding CARVYKTI's market reach.
Janssen and Legend Biotech are actively working to improve manufacturing processes to reduce vein-to-vein time and address supply chain bottlenecks. Furthermore,
ongoing trials, such as
CARTITUDE-4, are exploring CARVYKTI's use in earlier lines of therapy, which could significantly expand its patient base and market potential. If successful in earlier settings, CARVYKTI could shift the treatment paradigm for multiple myeloma and
increase adoption among physicians and
healthcare providers.
Despite its promising clinical profile, CARVYKTI faces challenges related to
reimbursement and healthcare infrastructure. The
high cost of CAR-T therapies often limits patient access, particularly in markets with strict reimbursement policies. Moreover, the
complex nature of CAR-T administration, which requires specialized centers and trained personnel, adds to the barriers. However,
continued clinical success, regulatory support, and improvements in manufacturing and delivery infrastructure could strengthen CARVYKTI's market position and establish it as a cornerstone therapy in the multiple myeloma treatment landscape.
Dive deeper to get more insight into CARVYKTI's strengths & weaknesses relative to competitors @
CARVYKTI Market Drug Report
Table of Contents
Related Reports
Relapsing Refractory Multiple Myeloma Market
Relapsing Refractory Multiple Myeloma Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key RRMM companies including
AbbVie, Genentech, Amgen, Onyx Therapeutics Inc., Bristol-Myers Squibb, MedImmune LLC, Novartis Pharmaceuticals, Incyte Corporation, Takeda, among others.
Relapsing Refractory Multiple Myeloma Pipeline
Relapsing Refractory Multiple Myeloma Pipeline Insight
– 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key RRMM companies, including
Bristol-Myers Squibb, I-MAB Biopharma, Pfizer, Arcellx, Gilead Sciences, Novartis, Array Biopharma, Hrain Biotechnology Co., Ltd., Cartesian Therapeutics, Xencor, Takeda, Sorrento Therapeutics, Heidelberg Pharma AG, Ichnos Sciences, Allogene Therapeutics, Harpoon Therapeutics, Cellectis, Poseida Therapeutics, Regeneron Pharmaceuticals, ONK Therapeutics, TeneoOne, iTeos Therapeutics, Oricell Therapeutics, Anaveon AG, Luminary Therapeutics, Seagen Inc., Trillium Therapeutics Inc., Virtuoso BINco, Inc., Seagen Inc., Trillium Therapeutics Inc., among others.
Multiple Myeloma Market
Multiple Myeloma Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key multiple myeloma companies including
Sanofi, Karyopharm Therapeutics, AbbVie, Takeda Pharmaceutical, Celgene, Bristol-Myers Squibb, RAPA Therapeutics, Pfizer, Array Biopharma, Cellectar Biosciences, BioLineRx, Celgene, Aduro Biotech, ExCellThera, Janssen Pharmaceutical, Precision BioSciences, Takeda, Glenmark (Ichnos Sciences SA), Poseida Therapeutics, Molecular Partners AG, Chipscreen Biosciences, AbbVie, Genentech (Roche), Janssen Biotech, Nanjing Legend Biotech, Merck Sharp & Dohme Corp., among others.
Multiple Myeloma Pipeline
Multiple Myeloma Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key multiple myeloma companies, including
CASI Pharmaceuticals, Carsgen Therapeutics, Cartesian Therapeutics, Gracell Biotechnology Shanghai Co., Ltd., Sorrento Therapeutics, TeneoOne, Karyopharma Therapeutics, Arcellx, Poseida Therapeutics, Ichnos Sciences, Nerviano Medical Sciences, Bristol Myers Squib, Ascentage Pharma, Ionis Pharmaceuticals, Chongqing Precision Biotech Co., Ltd., CRISPR Therapeutics, AstraZeneca, IGM Biosciences, Novartis, GlaxoSmithKline, Innovent Biologics, Keymed Biociences, Starton Therapeutics, Takeda, Fate Therapeutics, Gilead Sciences, Jiangsu Chia Tai Fenghai Pharmaceutical Co., Ltd., Janssen Pharmaceutical, Nanjing IASO Biotechnology Co., Ltd., GPCR Therapeutics, Chimerix, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve
.
Contact Us
Shruti Thakur
[email protected]
+14699457679
Logo:
SOURCE DelveInsight Business Research, LLP
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED
Clinical ResultImmunotherapyOrphan DrugDrug ApprovalPhase 3
26 Nov 2024
Investors drove Poseida Therapeutics\' share price up more than 200% in premarket trading. \n Roche has struck a $1.5 billion deal to buy Poseida Therapeutics. The takeover will establish off-the-shelf cell therapies, which Roche has said can democratize CAR-Ts, as a new core capability at the drugmaker.Poseida partnered with Roche in 2022, accepting a $110 million upfront in return for licenses on two cell therapy candidates and options on other prospects. The deal, which included $6 billion in milestones, moved Roche deeper into cell therapies. Roche stayed on the sidelines in the early years of excitement about CAR-T but has struck deals to build out a cell therapy presence in recent years.The Poseida takeover, which was announced alongside a major setback for the Swiss drugmaker’s TIGIT program, marks a step up in Roche’s pursuit of the cell therapy market. Roche has agreed to buy the biotech for $9 a share in cash. A further $4 a share is tied to the achievement of certain milestones. The total equity value of the deal is $1.5 billion.The outlay will give Roche control of a pipeline of cell therapies and the underlying technology platform. Roche already has licenses to Poseida’s two lead blood cancer programs. The biotech moved a CAR-T cell therapy against BCMA into the clinic in multiple myeloma 2022 and began testing a CD19xCD20 prospect in humans this year. Roche also has an option on Poseida’s CD70-directed acute myeloid leukemia asset. Buying Poseida will give Roche full ownership of those assets, plus a BCMAxCD19 prospect that Poseida is developing in multiple myeloma and autoimmune diseases and a clutch of solid tumor candidates. The solid tumor work, which includes a collaboration with Astellas, is led by a MUC1-C candidate that moved into the clinic in 2022.Other companies are aiming cell therapies at the same targets but Roche is betting Poseida’s nonviral platform will set its candidates apart. The platform is designed to generate off-the-shelf CAR-T therapies that are rich in T stem cell memory cells, a long-lived, self-replicating cell type that Poseida identified as a way to improve safety and efficacy.Charles Fuchs, M.D., global head of oncology and hematology drug development at Genentech and Roche, set out the case for the platform at the company’s Pharma Day event in September. Fuchs said around 20% of patients eligible for CAR-T receive cell therapy. Poseida’s donor-derived healthy stem cell memory T cells could allow Roche to “truly scale CAR-T therapy to a much broader population,” Fuchs said.The Roche exec also highlighted Poseida\'s gene editing tools, including “piggyback insertion technology, which allows for the delivery of multiple CARs in a single step with great efficiency and without the need of viral delivery systems.” Another Poseida technology “allows for multiple knock-in and knock-outs” to prevent graft-versus-host and host-versus-graft responses, Fuchs said. Poseida has struggled to get investors excited about the technology. The biotech went public in 2020 and briefly traded above $15 a share. Since then, Poseida’s share price has fallen more than 80% to close at $2.86 before news of the Roche deal broke.Investors drove the biotech’s share price up more than 200% in premarket trading. At $9.20, the stock is trading above the upfront offered by Roche but well below the price including success-based payments.
Cell TherapyAcquisitionImmunotherapy
11 Oct 2024
Image caption: Shutterstock/AuthenticVision
Cell therapies stand at the frontier of medicine and hold the potential to potentially cure diseases, said Angela Vollstedt, global director of Cell & Gene Therapies at
Novartis
in her opening remarks during a talk at CPHI Europe.
Vollsetdt was speaking at the industry conference, which took place 8–10 October in Milan, Italy. According to Vollstedt, the venture capitalist (VC) community is the one driving cell therapies like chimeric antigen receptor (CAR)-Ts in clinical development although there is still a lot of untapped potential and opportunities for growth.
“Only 30% of patients end up receiving CAR-Ts out of all eligible patients,” she said. Complex manufacturing procedures and slow bureaucratic referral systems extend timelines that make patients ineligible to receive these treatments, Vollstedt said. While the demand for cell therapies is rising, the supply of manufacturing capacities is lagging.
Some key cost drivers that pose a barrier to broad cell therapy access include the reliance on a highly skilled workforce, time consuming processes with open handling, and strict quality controls. Also, a limited number of approved treatment centers and sites for cell therapy administration, which require patients to travel, can be challenging.
There is a “complex inter-dependency of players” in the cell therapy manufacturing industry, which drives the inefficiencies and dictates the ability to serve patients in a timely manner, Vollstedt added.
See Also:
Resilience secures funds to enhance production of essential medicine components
Gilead signs agreements to facilitate access to HIV prevention drug
According to Vollstedt, “de-bottlenecking” the supply of cell therapies will be crucial to ensuring broader access if investments in the space happen.
Strategies to combat manufacturing inefficiencies
Firstly, the manufacturing of lentiviral vectors, the most common delivery method for transgene delivery, is usually outsourced, which adds further complexity to the manufacturing process. The quality, design, and compatibility of the vector process development is also a key issue because Vollstedt said it could ultimately “cost you your product in ways you will not be able to recover from”.
Then, patient batch variability at each timepoint of the patient journey should be considered. The process of apheresis needs to be standardised at every single point, Vollstedt said, and donor cell variability should be considered early into the manufacturing process because differences in cellular starting materials can impact product yield.
Additionally, investment in automated manufacturing platforms in a closed manufacturing system needs to be complemented by appropriate analytical testing panels to improve the process efficiency, she said. Limitations in treatment accessibility stem from a shortage of approved treatment centres and limited physician referrals due to low awareness and acceptance of CAR-T therapies. Alternative manufacturing models, such as
point-of-care manufacturing
need to be explored along with the establishment of more treatment centers to ensure centralisation for increased scale, she said.
Overall, strategies to achieve de-bottlenecking of supply chain and logistics should be implemented both in cell collection and transport and distribution. Additionally, post-collection handling and logistics should be standardized, according to Vollstedt, with the use of specialised shipping methods (cold-chain logistics) and national transport networks coupled with careful planning of delivery timelines to treatment centers.
For pharmaceutical companies developing cell therapies, go-to-market considerations should be part of development from their inception. For instance, automated processes to identify manufacturing procedure parameters, which are indicative of product quality, should be adopted from early development stages. Vollstedt emphasised that this might delay first-in-human trials, but it will eventually prove beneficial in later development stages and closer to commericalisation.
The goal is to democratise patient access to life-saving therapies through better supply chain and logistics, said Vollstedt, and for this innovation must be made accessible.
Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva
.
Editorial content is independently produced and follows the
highest standards
of journalistic integrity. Topic sponsors are not involved in the creation of editorial content.
Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva
.
Editorial content is independently produced and follows the
highest standards
of journalistic integrity. Topic sponsors are not involved in the creation of editorial content.
Free Whitepaper
Cell and gene therapies: Pipe dream to pipeline
The cell and gene industry is gaining momentum, with a new wave of therapies promising to transform the way doctors treat, and even cure, disease. In this report, Cytiva and GlobalData have collaborated to explore the rise of the cell and gene therapy industries, the current state of the market, present and future opportunities for advancement, and the challenges that lie ahead.
Thank you.
You will receive an email shortly. Please check your inbox to download the Whitepaper.
By Cytiva Thematic
By downloading this Whitepaper, you acknowledge that GlobalData may share your information with
Cytiva Thematic
and that your personal data will be used as described in their
Privacy Policy

Cell TherapyGene Therapy
100 Deals associated with CARTs(University of Pennsylvania)
Login to view more data
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Adenocarcinoma | Preclinical | United States | 11 Jan 2023 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
No Data | |||||||
Login to view more data
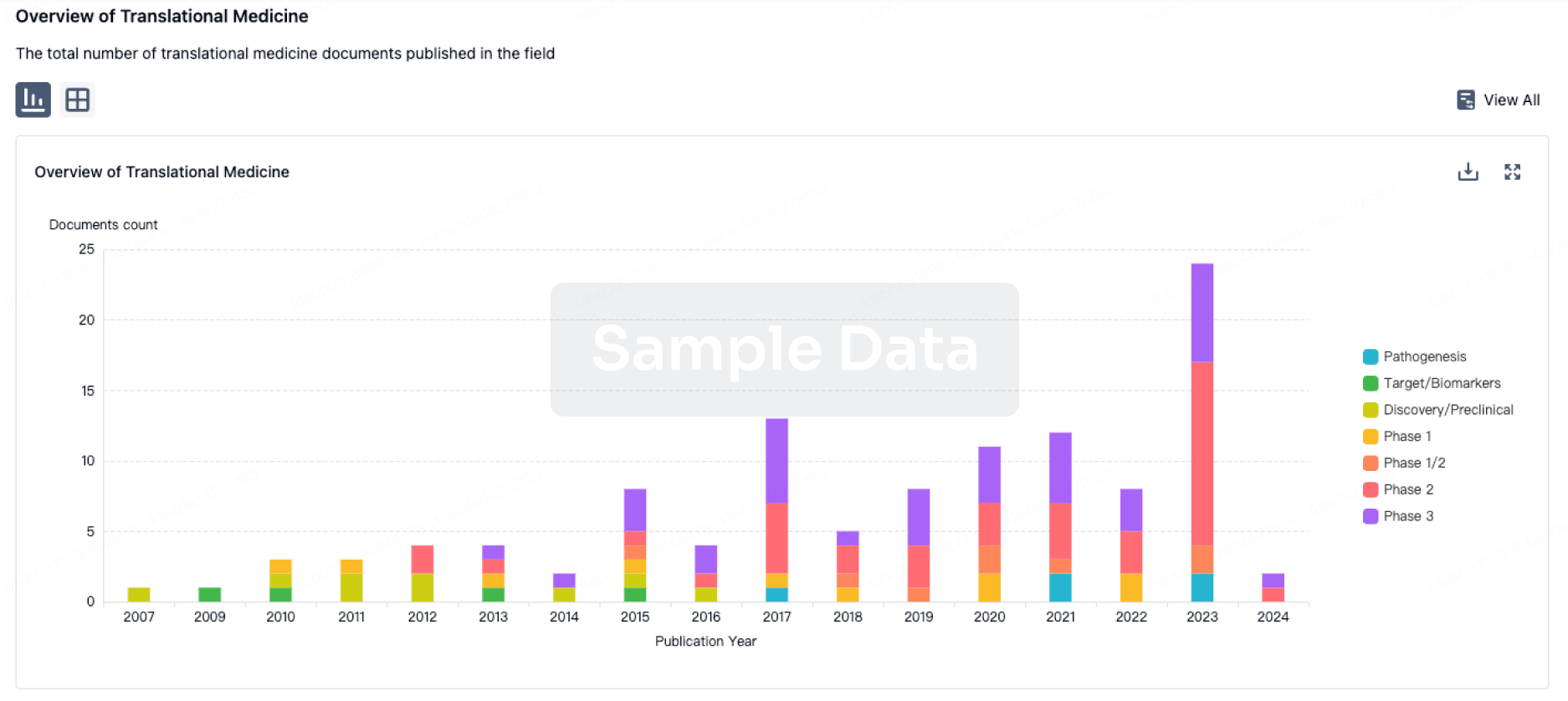
Translational Medicine
Boost your research with our translational medicine data.
login
or
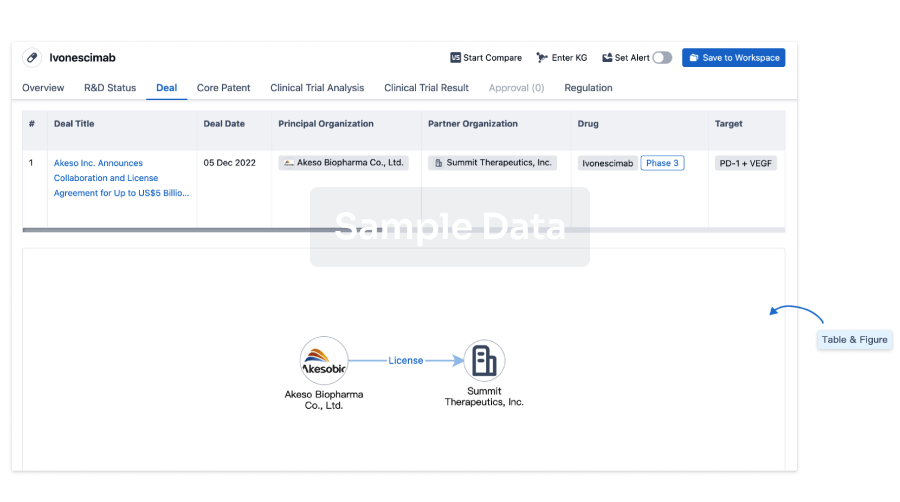
Deal
Boost your decision using our deal data.
login
or
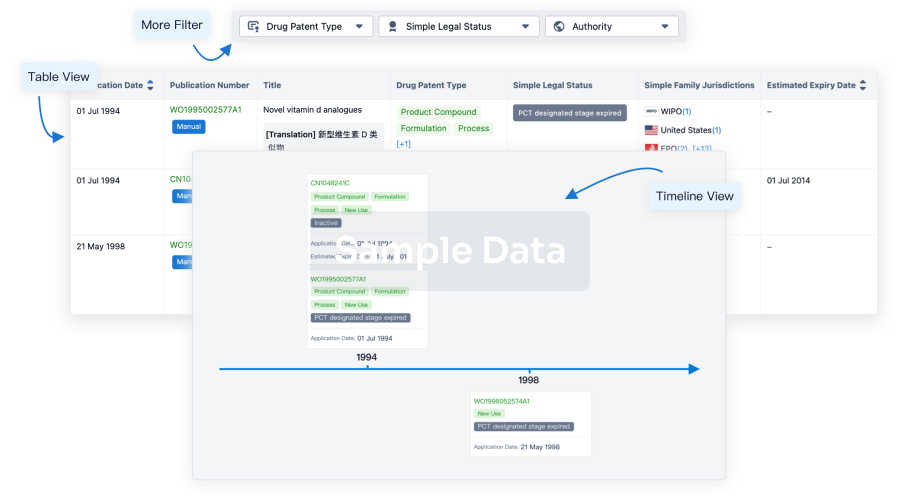
Core Patent
Boost your research with our Core Patent data.
login
or
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
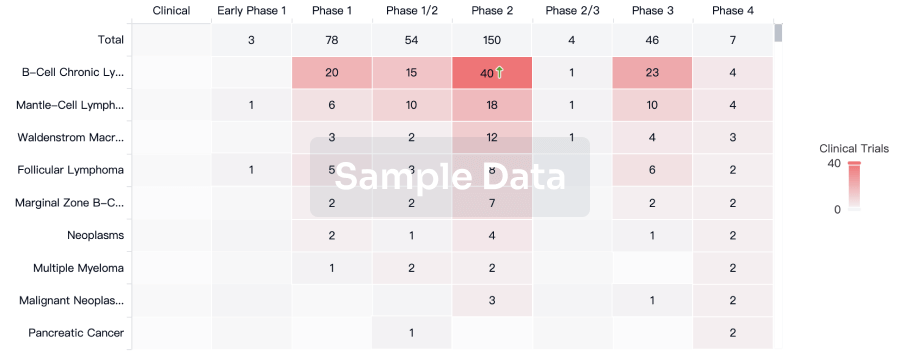
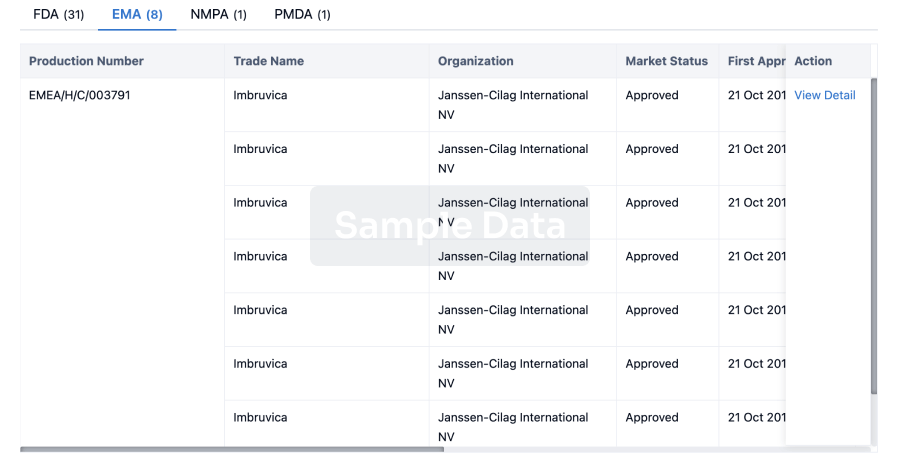
Approval
Accelerate your research with the latest regulatory approval information.
login
or
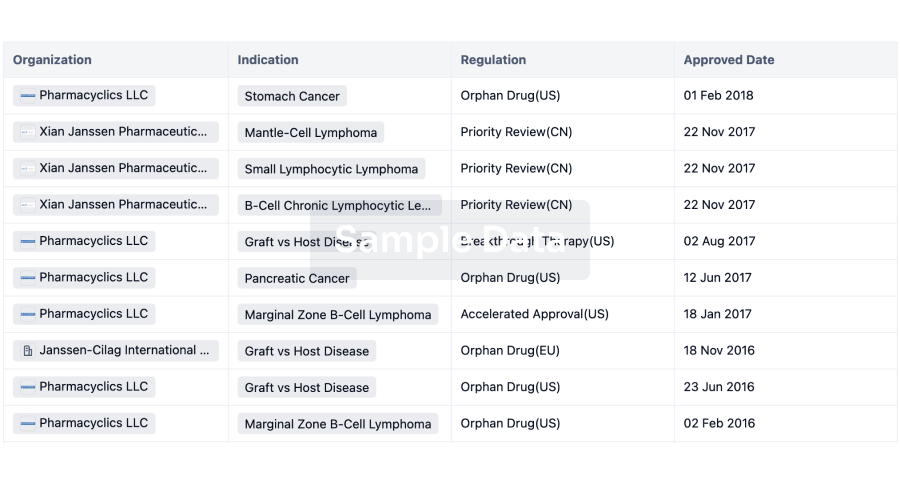
Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free
