Request Demo
Last update 20 Mar 2025
Ferric Sulfate
Last update 20 Mar 2025
Overview
Basic Info
Drug Type Small molecule drug |
Synonyms- |
Target- |
Action- |
Mechanism- |
Therapeutic Areas- |
Active Indication- |
Inactive Indication- |
Originator Organization- |
Active Organization- |
Inactive Organization- |
License Organization- |
Drug Highest Phase- |
First Approval Date- |
Regulation- |
Related
10
Clinical Trials associated with Ferric SulfateIRCT20100125003168N8
Comparison of radiographic and clinical success rates of ferric sulfate, theracal, MTA, and Protooth in pulpotomy of primary molars: A double-blind clinical trial with 6- and 12-month follow-up.
Start Date19 Jan 2025 |
Sponsor / Collaborator |
NCT05545527
Neuroimaging Ancillary Study of the IV Iron RAPIDIRON Trial
As a follow-up to the RAPIDIRON Trial (NCT05358509), and in combination with the RAPIDIRON-KIDS Study (NCT05504863), this study will involve infants of RAPIDIRON Trial participants recruited at one site in Karnataka and is designed to implement a magnetic resonance imaging (MRI) protocol and incorporate neuroimaging measures. Implementation of this study will promote an understanding of the effects on fetal and neonatal brain development, including iron deposition in brain tissues, when a woman is treated for iron deficiency anemia (IDA) by either (a) providing her oral iron tablets and instructions for use; or (b) administering a single-dose IV iron infusion for the treatment of IDA during pregnancy.
Start Date21 Feb 2023 |
Sponsor / Collaborator |
NCT05504863
Effect of Iron Supplementation During Pregnancy on Neurodevelopmental Status of Babies
As a follow-up to the RAPIDIRON Trial (NCT05358509), this study will follow the previously randomized mothers as well as their offspring after birth to assess neurodevelopmental, hematologic, and health outcomes. The study's overarching goal is to determine if the offspring born to RAPIDIRON Trial mothers in the intravenous iron groups, compared to the oral iron group, will achieve superior neurodevelopment, iron stores, and growth at specific time points during the first three years of life. Differences will be assessed between offspring based on the iron deficiency anemia (IDA) treatment of the mother.
Start Date11 Oct 2022 |
Sponsor / Collaborator |
100 Clinical Results associated with Ferric Sulfate
Login to view more data
100 Translational Medicine associated with Ferric Sulfate
Login to view more data
100 Patents (Medical) associated with Ferric Sulfate
Login to view more data
159
Literatures (Medical) associated with Ferric Sulfate01 Aug 2024·Minerva Dental and Oral Science
Apoptotic effects of biodentine, calcium-enriched mixture (CEM) cement, ferric sulfate, and mineral trioxide aggregate (MTA) on human mesenchymal stem cells isolated from the human pulp of exfoliated deciduous teeth
Article
Author: Mohebbi Rad, Mahshid ; Saburi, Ehsan ; Nazemi Salman, Bahareh
01 Jul 2024·Indian Pediatrics
Comparative Efficacy of Ferrous, Ferric and Liposomal Iron Preparations for Prophylaxis in Infants
Article
Author: Konuksever, Dilek ; Özbek, Namik Yasar ; Kiliç, Betül Orhan
01 Jun 2024·Heliyon
The effect of ZnSO4 and Fe2(SO4)3 on the pyrolysis of cocoa shells: A tg-FTIR study
Article
Author: Vesga, Angie Xiomara ; Cuentas, Maria Fernanda ; Albis Arrieta, Alberto Ricardo
2
News (Medical) associated with Ferric Sulfate29 Jan 2024
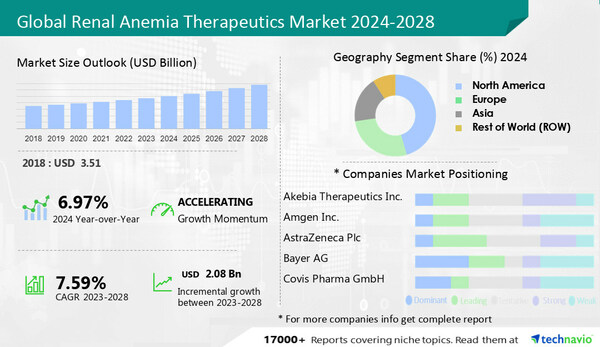
NEW YORK, Jan. 29, 2024 /PRNewswire/ -- The
renal anemia therapeutics market is estimated to grow by
USD 2.08 billion during 2023-2028, growing at a
CAGR of 7.59%. Akebia Therapeutics Inc and Amgen Inc to emerge as some of the major companies. Akebia Therapeutics Inc offers adadustat. The report includes information on the product launches, sustainability, and prospects of leading companies including Akebia Therapeutics Inc., Amgen Inc., AstraZeneca Plc, Bayer AG, Covis Pharma GmbH, CSL Ltd., Daiichi Sankyo Co. Ltd., Dr Reddys Laboratories Ltd., F. Hoffmann La Roche Ltd., FibroGen Inc., GlaxoSmithKline Plc, Japan Tobacco Inc., JCR Pharmaceticals Co. Ltd., Kirin Holdings Co. Ltd., Mitsubishi Chemical Group Corp., Pfizer Inc., Pharmacosmos AS, Sun Pharmaceutical Industries Ltd., Travere Therapeutics Inc., and Astellas Pharma Inc..
For Comprehensive details on the market size of historic period(2018 to 2022) and forecast period (2024-2028) - View the Free Sample report
Continue Reading
Technavio has announced its latest market research report titled Global Renal Anemia Therapeutics Market 2024-2028
The market is fragmented; the companies are competing with competitors and are trying to get greater market share. The market is growing, and chances of new entrants cannot be overlooked. The major companies have well-established economies of scale and presence and generally rely on positioning, technological advances, and the price of the products -
The report provides a full list of key companies, their strategies, and the latest developments. Buy Now
The growing geriatric population drives the growth. The prevalence of chronic diseases like CKD, is high among the aging population. There is a direct relationship between aging and susceptibility to infections, as it deteriorates the immune system. Apart from the impaired functioning of the immune system, the reduced functioning of organs also increases the chances of acquiring diseases such as CKD.
The growing adoption of biosimilars is an emerging trend shaping growth.
Side effects of oral administration of renal anemia drugs is a major challenge hindering growth.
Technavio has identified key trends, drivers, and challenges, which will help clients improve their strategies to stay ahead of their competitors. - View the Free Sample Report
The report includes competitive analysis, a proprietary tool to analyze and evaluate the position of companies based on their industry position score andperformance score. The competitive scenario categorizes companies based on various performance indicators. Some of the factors considered include the financial performance of companies over the past few years, growth strategies, product innovations, new product launches, investments, and growth in market share, among others.
Segmentation by Type
The growth by the
IV segment will be significant during the forecast period. The predominant segment is medications administered through the IV route, primarily attributed to the growing adoption and the convenience of absorption. Additionally, ferric carboxymaltose, sucrose, dextran, and other agents like ferumoxytol and isomaltoside contribute to this category.
Gain instant access to 17,000+ research reports.
Technavio's SUBSCRIPTION platform
Applications
The Renal Anemia Therapeutics Market is a dynamic landscape where medical interventions target conditions such as Chronic Kidney Disease (CKD) through a range of treatments. Erythropoiesis-Stimulating Agents (ESAs), including Darbepoetin Alfa and Epoetin Alfa, play a pivotal role in managing anemia associated with renal diseases. Innovative therapies like Roxadustat, a Hypoxia-Inducible Factor Prolyl Hydroxylase (HIF-PH) Inhibitor, showcase advancements in addressing anemia linked to CKD. Iron Supplements, both intravenous and oral, are essential components, alongside Ferric Citrate and Ferric Gluconate, contributing to comprehensive anemia management. The market encompasses diverse modalities, from Hemodialysis to Peritoneal Dialysis, recognizing the significance of tailored approaches.
Related Reports
The
hormone replacement therapy (HRT) market size is estimated to grow by USD 8.44 billion, at a CAGR of 6.67% between 2023 and 2028.
The
equine supplement products market size is estimated to grow by USD 20,352.14 at a CAGR of 4.19% between 2023 and 2028.
1 Executive Summary
2 Landscape
3 Sizing
4 Historic Size
5 Five Forces Analysis
6 Segmentation by Type
7 Segmentation by Distribution Channel
8 Customer Landscape
9 Geographic Landscape
10 Drivers, Challenges, and Trends
11 Company Landscape
12 Company Analysis
13 Appendix
About US
Technavio is a leading global technology research and advisory company. Their research and analysis focus on emerging trends and provide actionable insights to help businesses identify opportunities and develop effective strategies to optimize their positions. With over 500 specialized analysts, Technavio's report library consists of more than 17,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio's comprehensive coverage, extensive research, and actionable insights to identify opportunities in existing and potential markets and assess their competitive positions within changing scenarios.
Contact
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
Email: [email protected]
Website:
SOURCE Technavio
06 Jun 2022
NEW YORK, June 6, 2022 /PRNewswire/ -- Polaris Market Research recently published a research report on
"Intravenous Iron Drugs Market Share, Size, Trends, Industry Analysis Report, By Application (Chronic Kidney Disease, Inflammatory Bowel Disease, Cancer, Others), By Products (Iron Dextran, Iron Sucrose, Ferric Carboxymaltose, Others), By Region; Segment Forecast, 2021 - 2028" in its research database.
According to recent research study, the global intravenous iron drugs market size & share is expected to
grow at a CAGR of 8.6% between 2021 and 2028. The cold chain monitoring industry revenue of USD 2.22 billion in 2020 is
expected to grow up to USD 4.02 billion by 2028.
What is Intravenous Iron Drugs? How Big is Intravenous Iron Drugs Market?
Report Overview
Iron is an essential mineral that our bodies need for many biological functions such as synthesis of heme, energy metabolism, neurotransmitter production, formation of myoglobin, formation of collagen, and immune system function. The deficiency of iron can cause iron deficiency anemia (IDA). Iron supplements can help convert low iron levels or treat iron deficiency anemia. Intravenous iron drug supplementation is the process of delivering iron to the body through a needle into a vein. Patients unable to tolerate or absorb oral iron or suffering from heavy menses, celiac disease can take IV iron drugs.
Iron supplements are of two types: oral and intravenous. Intravenous iron drugs are superior compared to oral supplements as these lead to a higher and faster increase in iron and Hb levels. Other benefits of these drugs include increased energy and easier breathing. The growing demand for intravenous iron drugs owing to the rising number of research and development activities is fueling the growth of the global intravenous iron drugs market. Surging cases of Inflammatory Bowel Disease (IBD), and cancer around the globe have led to an increase in the need for intravenous iron drugs, which is expected to propel market growth
Request Sample Copy of "Intravenous Iron Drugs Market" Research Report @
The Reader Will Find the Following Key Points from This Research Document
Existing intravenous iron drugs market size and overview
Challenges and opportunities
Best regions and segments to target
Touchpoints and an opportunity breakdown within the value chain
The growth rate during the forecast period
Key factors driving the intravenous iron drugs market
Key market trends cracking up the growth of the market
Key vendors of the intravenous iron drugs market
Opportunities and threats faced by the existing vendors
Top Players in the Global Market Are:
AbbVie Inc.
Allergan
AMAG Pharmaceuticals
American Regent Inc.
Daiichi Sankyo Company Ltd. (American Regent. Inc.)
Fresenius Medical Care AG & Co. KGaA
Galenica Ltd.
Pharmacosmos A/S
Rockwell Medical Inc.
Sanofi
SHIELD THERAPEUTICS
Vifor Pharma Management Ltd
Request More Information on Top Market Players HERE
Intravenous Iron Drugs Market: Driving Factors
The launch of new formulations with innovative development and the rising incidences of chronic kidney diseases & anemia is expected to propel the intravenous iron drugs market growth. The growing research and activities, and increasing awareness of diagnosis and treatment of anemia are anticipated to drive the market growth over the forecast period. Rise in iron deficiency anemia in gynecology, oncology, and gastroenterology, noncompliance with oral therapy, and convenient access to IV iron dosages duel the demand for intravenous iron drugs. Moreover, the surge in prevalence of chronic diseases, such as cancer, inflammatory bowel diseases, diabetes, and acute and chronic infections is further boosting the significant demand for intravenous iron drugs. In addition, several government activities by the government and drug manufacturers are expected to accelerate the growth of the intravenous iron drugs market globally.
Directly Purchase a copy of report with TOC @
Intravenous Iron Drugs Market: Key Insights, Dynamics & Research Scope
Also Read: Press Release on Intravenous Iron Drugs Market Size Worth $4.02 Billion by 2028 | CAGR: 8.6%
Intravenous Iron Drugs Market: Segmentation
Insight by Product
On the basis of product, the market is segmented into iron dextran, iron sucrose, ferric carboxymaltose, and others. The Ferric carboxymaltose (FCM) segment is accounted for the largest market share in 2020 and is anticipated to continue its dominance in the intravenous iron drugs market in the forecast period. This growth can be attributed to growing applications in intravenous drug discovery, enhanced performance skills, and increasing cost-effectiveness. FCM is majorly used to treat diseases such as iron deficiency anemia, chronic kidney disease, heavy uterine bleeding, and inflammatory bowel disease. Thus these factors are contributing to the growth of this segment around the world which will boost the intravenous iron drug market. The others segment including ferric gluconate, iron isomaltoside, ferumoxytol drugs, and ferric pyrophosphate citrate is expected to generate significant growth over the foreseen period.
Insight by Application
Based on application, the market is categorized into chronic kidney disease, inflammatory bowel disease, cancer, and others. The chronic kidney disease segment witnessed the largest market share in 2020 owing to rising cases of chronic kidney disease (CKD) and the growing prevalence of iron deficiency anemia among CKD patients. People suffering from CKD are more likely to develop anemia compared to the general population. Thus, the rising cases of CKD worldwide are expected to increase demand for intravenous iron drugs, which will contribute to the segment growth.
Moreover, the cancer segment is projected to record the highest CAGR in the intravenous iron drug market due to the high incidence of cancer and rising IDA cases worldwide. The most common types of cancer that are associated with anemia include blood cancer, bone cancer, cervical cancer, colon cancer, and prostate cancer.
Inquire more about this report before purchase @
Covid-19 Impact
The recent outbreak of Covid-19 positively affected the intravenous ferrous drugs market across the world. This is because, Covid-19 caused an increase in chronic kidney diseases (CKD) and Acute Kidney Injury (AKI), which boost the market demand for intravenous iron drugs. According to the National Kidney Foundation, hospitalized Covid-19 patients are more likely to develop AKI in comparison to non- Covid patients.
Geographic Overview: Intravenous Iron Drugs Market
Based on geography, North America accounted for the largest market share in 2020 and is anticipated to continue its dominance in the global market during the forecasting period. The key factors contributing to this regional growth in the intravenous iron drugs market include growing approvals by the healthcare professionals and the launch of new formulated intravenous drugs. Also, the increasing incidences of gastrointestinal disorders, cancer, and chronic kidney diseases, along with the growing awareness regarding female health are some of the factors driving the market growth in the region.
Further, the Asia Pacific market is projected to witness the fastest growth rate over the forecast period owing to increasing investment by governments, developing healthcare infrastructure, and the rising presence of key players in Asia Pacific countries. In addition, the increasing prevalence of IDA in emerging nations such as China and India also led to the growth of the intravenous iron drugs market.
Browse the Detail Report
"Intravenous Iron Drugs Market Share, Size, Trends, Industry Analysis Report, By Application (Chronic Kidney Disease, Inflammatory Bowel Disease, Cancer, Others), By Products (Iron Dextran, Iron Sucrose, Ferric Carboxymaltose, Others), By Region; Segment Forecast, 2021 - 2028" with in-depth TOC:
For Immediate Purchase OR Media Enquiry, Please Mail At: [email protected]
The market is primarily segmented on the basis of product, application, and region.
Intravenous Iron Drugs Market: By Product Outlook
Iron Dextran
Iron Sucrose
Ferric Carboxymaltose
Others
Intravenous Iron Drugs Market: By Application Outlook
Chronic Kidney Disease
Inflammatory Bowel Disease
Cancer
Others
Key Questions Answered:
Who are the leading key players, and what are their critical business plans in the global intravenous iron drugs market?
What are the critical concerns of the five forces analysis?
What are the different prospects and threats faced by the dealers?
What are the strengths and weaknesses of the key vendors?
What are the main trends that have a positive impact on the growth of the global intravenous iron drugs market?
What are the growth opportunities that could emerge in the industry in the coming years?
Browse More Related Reports:
Organ Preservation Market Share, Size, Trends, Industry Analysis Report, By Solution; By Technique; By End-User (Organ Transplant Centers, Hospitals, Specialty Clinics); By Organ Type (Kidney, Liver, Lung, Heart, Pancreas); By Region; Segment Forecast, 2022 – 2030
Microplate Systems Market Share, Size, Trends, Industry Analysis Report, By Product; By Application (Drug Discovery, Clinical Diagnostics Genomics & Proteomics Research Others); By End-use; By Region; Segment Forecast, 2022 – 2030
Population Health Management Market Share, Size, Trends, Industry Analysis Report, By Component (Software, Services); By End-Use (Healthcare Providers, Healthcare Payers, Others); By Mode Of Delivery; By Region; Segment Forecast, 2022 – 2030
Pharmacovigilance Market Share, Size, Trends, Industry Analysis Report, By Service, By Product Life Cycle, By Type, By Process Flow, By Therapeutic Area, By End-Use, By Region; Segment Forecast, 2022 – 2030
Medical Fiber Optics Market Share, Size, Trends, Industry Analysis Report, By Fiber Type (Single Mode Optical, Multimode Optical), By Application (Endoscopic Imaging, Laser Signal Delivery, Biomedical Sensing, Illumination, Others) By Region; Segment Forecast, 2022 - 2030
About Polaris Market Research
Polaris Market Research is a global market research and consulting company. The company specializes in providing exceptional market intelligence and in-depth business research services for PMR's clientele spread across different enterprises. We at Polaris are obliged to serve PMR's diverse customer base present across the industries of healthcare, technology, semi-conductors and chemicals among various other industries present around the world. We strive to provide PMR's customers with updated information on innovative technologies, high growth markets, emerging business environments and latest business-centric applications, thereby helping them always to make informed decisions and leverage new opportunities. Adept with a highly competent, experienced and extremely qualified team of experts comprising SMEs, analysts and consultants, we at Polaris endeavor to deliver value-added business solutions to PMR's customers.
Contact:
Likhil G
30 Wall Street
8th Floor,
New York City, NY 10005,
United States
Phone: +1-929 297-9727
Email: [email protected]
Web:
Follow US: LinkedIn
| twitter
SOURCE Polaris Market Research
100 Deals associated with Ferric Sulfate
Login to view more data
R&D Status
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
No Data | |||||||
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or
Deal
Boost your decision using our deal data.
login
or
Core Patent
Boost your research with our Core Patent data.
login
or
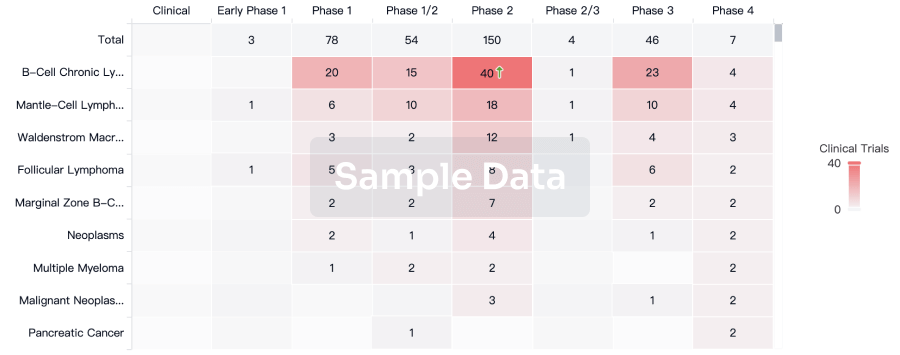
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
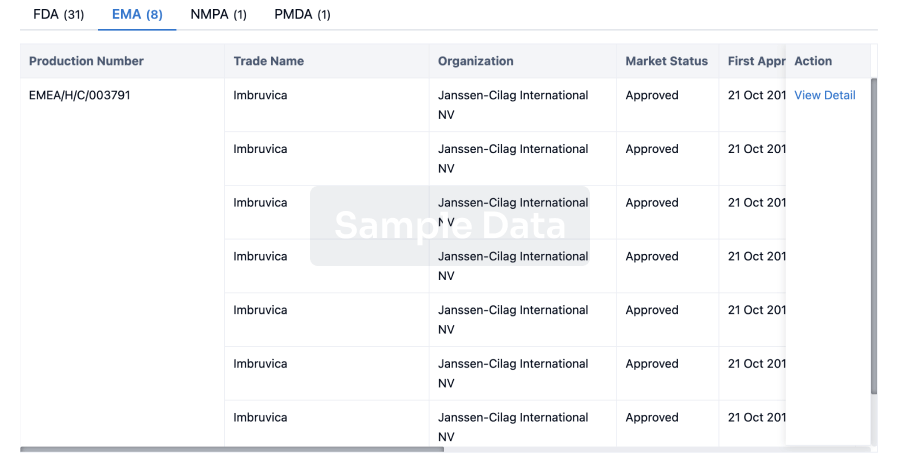
Approval
Accelerate your research with the latest regulatory approval information.
login
or
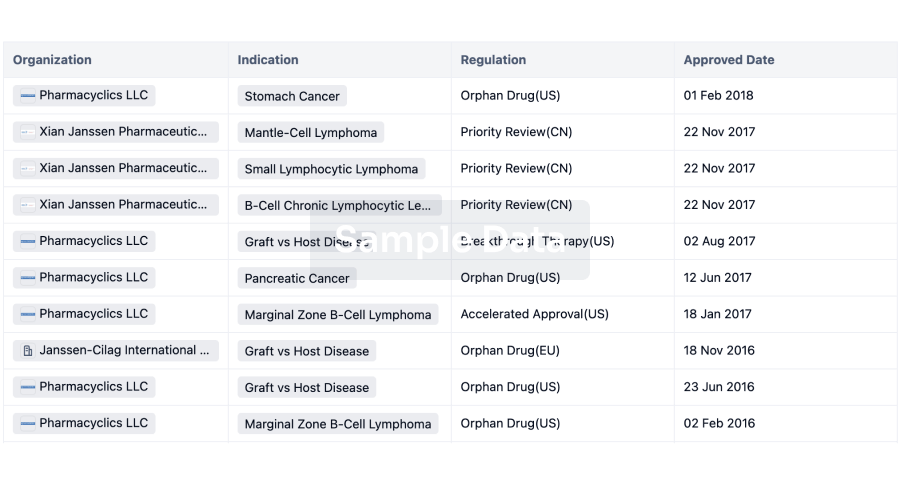
Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free

