Request Demo
Last update 26 Jan 2026
Insulin aspart biosimilar(Gan & Lee Pharmaceuticals Co., Ltd.)
Last update 26 Jan 2026
Overview
Basic Info
Drug Type Biosimilar, Peptide Hormone |
Synonyms Insulin aspart Biosimilar (Gan & Lee Pharmaceuticals Co., Ltd.), 门冬胰岛素30生物类似物 (甘李药业), 门冬胰岛素生物类似药(Gan & Lee Pharmaceuticals Co., Ltd.) + [1] |
Target |
Action agonists |
Mechanism INSR agonists(Insulin receptor agonists) |
Therapeutic Areas |
Active Indication |
Inactive Indication- |
Originator Organization |
Active Organization |
Inactive Organization- |
License Organization- |
Drug Highest PhaseApproved |
First Approval Date China (05 Jun 2020), |
Regulation- |
Login to view timeline
Structure/Sequence
Sequence Code 4857

The sequence is quoted from: *****
Sequence Code 31953

The sequence is quoted from: *****
Related
6
Clinical Trials associated with Insulin aspart biosimilar(Gan & Lee Pharmaceuticals Co., Ltd.)CTR20232521
在口服降糖药控制不佳(A部分)或既往使用基础/预混胰岛素控制不佳(B部分)的2型糖尿病患者中比较GZR101注射液与德谷门冬双胰岛素注射液(诺和佳®)的疗效、耐受性和安全性的多中心II期临床研究
[Translation] A multicenter phase II clinical study to compare the efficacy, tolerability and safety of GZR101 injection and degludec insulin aspart injection (Truojia®) in patients with type 2 diabetes who are poorly controlled by oral hypoglycemic drugs (Part A) or have been poorly controlled with basal/premixed insulin (Part B).
主要目的
A部分:对比GZR101注射液和德谷门冬双胰岛素注射液(诺和佳®)每日注射给药一次治疗16周后的疗效、安全性和耐受性。B部分:对比每日一次GZR101注射液联合每日一次门冬胰岛素和每日两次德谷门冬双胰岛素注射液(诺和佳®)治疗16周后的疗效、安全性和耐受性。
次要目的
研究GZR101注射液每日注射给药一次,治疗16周的GZR33(GZR101成分之一,在研超长效基础胰岛素类似物)和门冬胰岛素(GZR101成分之一,已上市速效胰岛素)的药代动力学(PK)特征。
[Translation]
Main objective
Part A: To compare the efficacy, safety and tolerability of GZR101 injection and insulin degludec aspart injection (Novogam®) after 16 weeks of treatment with once-daily injection. Part B: To compare the efficacy, safety and tolerability of GZR101 injection once daily combined with insulin degludec aspart injection once daily and insulin degludec aspart injection twice daily (Novogam®) after 16 weeks of treatment.
Secondary objective
To study the pharmacokinetic (PK) characteristics of GZR33 (one of the components of GZR101, an ultra-long-acting basal insulin analog under development) and insulin degludec aspart (one of the components of GZR101, a rapid-acting insulin already on the market) after 16 weeks of treatment with GZR101 injection once daily.
Start Date21 Nov 2023 |
Sponsor / Collaborator |
NCT04237129
A Glucose Clamp Trial Investigating The Biosimilarity of Gan & Lee Insulin Aspart Injection (Insulin Aspart 100 U/ml) With US and EU Insulin Aspart Comparator Products (NovoLog®/NovoRapid®) in Healthy Male Subjects
Primary objective:
To demonstrate pharmacokinetic (PK) and pharmacodynamic (PD) equivalence of Gan & Lee Insulin Aspart Injection with both EU-approved NovoRapid® and US-licensed NovoLog® (Reference Products) in healthy male subjects
Secondary objectives:
To compare the PK and PD parameters of the three insulin aspart preparations
To evaluate the single dose safety and local tolerability of the three insulin aspart preparations
To demonstrate pharmacokinetic (PK) and pharmacodynamic (PD) equivalence of Gan & Lee Insulin Aspart Injection with both EU-approved NovoRapid® and US-licensed NovoLog® (Reference Products) in healthy male subjects
Secondary objectives:
To compare the PK and PD parameters of the three insulin aspart preparations
To evaluate the single dose safety and local tolerability of the three insulin aspart preparations
Start Date27 Aug 2019 |
Sponsor / Collaborator |
CTR20131031
多中心、随机比较进口同类产品(诺和锐)与门冬胰岛素(锐秀霖)联合二甲双胍治疗糖尿病的有效性和安全性
[Translation] A multicenter, randomized comparison of the efficacy and safety of an imported similar product (NovoRapid) and insulin aspart (Radoxil) combined with metformin in the treatment of diabetes mellitus
主要目的:根据HbA1c的情况,判断锐秀霖是否疗效非劣于诺和锐;次要目的:比较锐秀霖治疗组和诺和锐治疗组与基线相比达到目标HbAlc<7.0%,和≤6.5%的受试者百分比、餐后2h血糖及空腹血糖的变化等
[Translation]
Primary purpose: To determine whether the efficacy of Resolv is non-inferior to that of NovoRapid based on the HbA1c situation; Secondary purpose: To compare the percentage of subjects reaching the target HbAlc <7.0% and ≤6.5% in the Resolvv and NovoRapid treatment groups compared with baseline, and the changes in 2h postprandial blood glucose and fasting blood glucose, etc.
Start Date- |
Sponsor / Collaborator- |
100 Clinical Results associated with Insulin aspart biosimilar(Gan & Lee Pharmaceuticals Co., Ltd.)
Login to view more data
100 Translational Medicine associated with Insulin aspart biosimilar(Gan & Lee Pharmaceuticals Co., Ltd.)
Login to view more data
100 Patents (Medical) associated with Insulin aspart biosimilar(Gan & Lee Pharmaceuticals Co., Ltd.)
Login to view more data
3
News (Medical) associated with Insulin aspart biosimilar(Gan & Lee Pharmaceuticals Co., Ltd.)15 Oct 2024
BEIJING, China I October 15, 2024 I
Recently, Gan & Lee Pharmaceuticals (Gan & Lee, Shanghai Stock Exchange: 603087) announced that three of its independently developed drugs — the bi-weekly GLP-1 receptor agonist GZR18 injection, the once-weekly basal insulin analog GZR4 injection, and the premixed dual insulin analog GZR101 injection — successfully achieved positive results in three Phase 2 clinical trials conducted in adult patients with Type 2 diabetes (T2D) in China. In these studies, Gan & Lee’s three innovative drugs demonstrated superior or comparable efficacy in lowering glycated hemoglobin (HbA1c) compared to the respective positive comparator drugs separately.
Gan & Lee Pharmaceuticals’ Diabetes Product Portfolio Diagram
Statement:
1. GZR18, GZR4, and GZR101 are investigational drugs that have not yet been approved in China.
2. Gan & Lee Pharmaceuticals does not recommend the use of any unapproved drugs/indications.
GZR18 Injection: a Phase 2 Study evaluate the Efficacy and Safety of GZR18 Injective vs. Semaglutide (Ozempic
®
) in Chinese Patients with Type 2 Diabetes
The Phase 2b clinical trial (CTR20232069) is a multicenter, randomized, open-label study comparing the efficacy and safety of GZR18 injection versus semaglutide (Ozempic
®
) in Chinese adult T2D patients with poor glycemic control on lifestyle interventions and/or unregulated use of anti-diabetic drugs, and on oral anti-diabetic treatment for at least three months. A total of 264 subjects were recruited across 25 clinical centers in China. Patients were randomized to receive bi-weekly GZR18 injections (12 mg, 18 mg, or 24 mg) or once-weekly 24 mg GZR18 injections, or 1 mg semaglutide for 24 weeks, including the dose-escalation period. The primary efficacy endpoint was the change in HbA1c from baseline after 24 weeks of continuous treatment. The subjects had a mean diabetes history of 11-18 years, and baseline HbA1c ranged from 8.28% to 8.56%.
After 24 weeks of treatment, the mean HbA1c reduction from baseline in the GZR18 groups was as follows: 1.87% (12 mg, bi-weekly), 2.28% (18 mg, bi-weekly), 1.94% (24 mg, bi-weekly), and 2.32% (24 mg, once-weekly), all higher than the semaglutide group (1.60% reduction)*. Additionally, in treatment-naïve patients with poor glycemic control on lifestyle interventions, the HbA1c reduction in the bi-weekly GZR18 injection group reached 2.98%, compared to 2.04% in the semaglutide group, with a significant HbA1c reduction (p < 0.05)*. After 24 weeks of treatment, patients in the bi-weekly GZR18 group experienced a maximum weight loss of 5.42 kg, compared to 3.25 kg in the semaglutide group*. GZR18 also greatly improved fasting glucose, blood pressure, and lipid levels, etc, providing comprehensive benefits for diabetic patients. GZR18 was generally well-tolerated, with safety and tolerability consistent with known GLP-1 receptor agonists. The most common adverse events were gastrointestinal-related, and no severe hypoglycemic events were observed.
GZR4 Injection: a Phase 2 Study Comparing the Efficacy and Safety of GZR4 injection vs. insulin degludec (Tresiba
®
) in Chinese Patients with Type 2 Diabetes
The Phase 2 trial (CTR20232431) was a multicenter, randomized, open-label, parallel-group study evaluating the efficacy, tolerability and safety of once-weekly GZR4 injections versus once-daily insulin degludec (Tresiba
®
) in 83 T2D patients with poor glycemic control on oral antidiabetic drugs (Part A) and 96 T2D patients with poor glycemic control on a combination of oral antidiabetic drugs and basal insulins (Part B).
In part A, the mean HbA1c reduction of once-weekly GZR4 injection was 1.50%, comparable to insulin degludec’s reduction of 1.48%. In part B, GZR4 injection demonstrated a superior HbA1c reduction of 1.26%, compared to -0.87% for insulin degludec (treatment difference: -0.38%, p < 0.01)*. In addition, improvements in time-in-range (TIR) were comparable between GZR4 and insulin degludec. In this study, GZR4 injection showed good safety and tolerability, with no severe hypoglycemic events observed.
GZR101 Injection: a Phase 2 Study Comparing the Efficacy and Safety of GZR101 injection vs. insulin degludec/insulin aspart (Ryzodeg
®
) in Chinese Patients with Type 2 Diabetes
The Phase 2 clinical trial (CTR20232431) is a multicenter, randomized, open-label, parallel-controlled, treat-to-target Phase 2 study. Part A of the study aimed to compare the efficacy, safety, and tolerability of GZR101 injection administered once-daily versus insulin degludec/insulin aspart (Ryzodeg
®
) administered once-daily over 16 weeks in 62 patients with uncontrolled T2D on oral anti-diabetic drugs. In Part B, the study compared the efficacy, safety, and tolerability of GZR101 injection combined with insulin aspart versus insulin degludec/insulin aspart (Ryzodeg
®
) administered twice-daily over 16 weeks in 91 patients with uncontrolled T2D on basal/premixed insulin.
In Part A, the HbA1c levels in the once-daily GZR101 injection group decreased by 1.56%, superior to the -1.31% reduction in the once-daily insulin degludec/insulin aspart group (treatment difference: -0.24%). In Part B, GZR101 injection combined with insulin aspart achieved HbA1c reductions of -1.64% (once-daily insulin aspart) and -1.68% (twice-daily insulin aspart), both higher than the -1.59% reduction in the twice-daily insulin degludec/insulin aspart group (treatment differences of -0.06% and -0.09%, respectively)*. Moreover, GZR101 injection demonstrated comparable efficacy to insulin degludec/insulin aspart in controlling fasting and postprandial blood glucose, as well as improving time-in-range (TIR). In this trial, GZR101 injection showed good safety and tolerability. The estimated incidence of hypoglycemic events in both groups did not show statistically significant differences during the study.
*The trial results are presented as least squares means (LS mean).
Note: severe hypoglycemia is defined as Grade 3 hypoglycemia (serious events without specific plasma glucose boundaries, with conscious and/or somatic changes, hypoglycemia requiring help from others).
Dr. Zhong-ru Gan, Chairman of Gan & Lee Pharmaceuticals, stated:
“The positive results achieved by GZR18, GZR4, and GZR101 in Phase 2 clinical trials mark an important milestone in improving the current landscape of diabetes treatment. These results demonstrate that our three products provide better glycemic control compared to similar antidiabetic drugs.
Over the years, we have successfully launched a full range of insulin products, from human insulin to insulin analogs, covering both basal and mealtime insulin, in the hope of comprehensively meeting the treatment needs of different diabetes patients. With recent breakthroughs in our research and development, we have also expanded our product portfolio with oral antidiabetic drugs, filling a gap in this critical area.
Focusing on the frontiers of the endocrine field, we are actively promoting the development of new drugs, aiming to achieve comprehensive management of chronic metabolic diseases, including diabetes and obesity. Gan & Lee’s product portfolio currently covers next-generation insulin based on the extension to emerging metabolic disease targets, such as GLP-1 receptor agonists, supporting patients throughout their treatment journey with a view to driving changes in the management of diabetes and metabolic diseases and improving the quality of life of patients.”
Forward-Looking Statements:
These forward-looking statements are based on expectations and assumptions as of the date of this release. Due to various factors, actual results may differ significantly. We do not undertake any obligation to update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
Reference
1. WANCAI XING, WEI CHEN, YINING ZHANG, JING GAO, ANSHUN HE, JUN ZHANG, YING DENG, FANGKAI XUE, YONGCHUN WANG, HAO FU, RUNYAO ZHANG, JINHUI HUANG, ZHONGRU GAN; 823-P: Molecular and Pharmacological Properties of GZR4, a Once-Weekly Insulin Analog.
Diabetes
14 June 2024; 73 (Supplement_1): 823–P.
https://doi.org/10.2337/db24-823-P
About Gan & Lee
Gan & Lee Pharmaceuticals developed the first Chinese domestic insulin analog. Currently, Gan & Lee has six core insulin products, including five insulin analog varieties: long-acting glargine injection (Basalin
®
), fast-acting lispro injection (Prandilin™), fast-acting aspart injection (Rapilin
®
), mixed protamine zinc lispro injection (25R) (Prandilin™25), aspart 30 injection (Rapilin
®
30), and one human insulin injection – mixed protamine human insulin injection (30R) (Similin
®
30). The company has two approved medical devices in China, namely reusable insulin injection pen (GanleePen), and disposable pen needle (GanleeFine
®
).
In China’s 2024 National Insulin-Specific Centralized Procurement, Gan & Lee Pharmaceuticals ranked first among all selected companies in terms of procurement demand for insulin analogs. The company is also making strides in international markets, with the disposable pen needle (GanleeFine
®
) approved by the US Food and Drug Administration (FDA) in 2020 and received GMP inspection approval from the European Medicines Agency (EMA) in 2024. These achievements significantly boost Gan & Lee’s competitiveness in both international and domestic markets.
In the future, Gan & Lee will strive for comprehensive coverage in diabetes treatment. Moving forward with its mission to become a world-class pharmaceutical company, Gan & Lee will also actively develop new chemical entities and biological drugs, focusing on treatments for metabolic diseases, cardiovascular diseases, and other therapeutic areas.
Further Information:
BPRD@ganlee.com
(Media)
BD@ganlee.com
(Business Development)
info.medical@ganlee.com
(Medical Information)
SOURCE:
Gan & Lee Pharmaceuticals
Clinical ResultPhase 2Drug Approval
15 Oct 2024
After 24 weeks of treatment in patients with Type 2 diabetes, the bi-weekly GLP-1 receptor agonist GZR18 injection showed superior efficacy in lowering HbA1c and body weight compared to semaglutide (Ozempic®).
After 16 weeks of treatment in patients with Type 2 diabetes, the once-weekly insulin analog GZR4 injection demonstrated superior HbA1c reduction in patients with uncontrolled diabetes on basal insulins, compared to insulin degludec (Tresiba®).
After 16 weeks of treatment in patients with Type 2 diabetes, the premixed dual insulin analog GZR101 injection showed superior efficacy in reducing HbA1c and postprandial glucose compared to insulin degludec/insulin aspart (Ryzodeg®).
BEIJING, Oct. 15, 2024 /PRNewswire/ -- Recently, Gan & Lee Pharmaceuticals (Gan & Lee, Shanghai Stock Exchange: 603087) announced that three of its independently developed drugs — the bi-weekly GLP-1 receptor agonist GZR18 injection, the once-weekly basal insulin analog GZR4 injection, and the premixed dual insulin analog GZR101 injection — successfully achieved positive results in three Phase 2 clinical trials conducted in adult patients with Type 2 diabetes (T2D) in China. In these studies, Gan & Lee's three innovative drugs demonstrated superior or comparable efficacy in lowering glycated hemoglobin (HbA1c) compared to the respective positive comparator drugs separately.
Gan & Lee Pharmaceuticals' Diabetes Product Portfolio Diagram
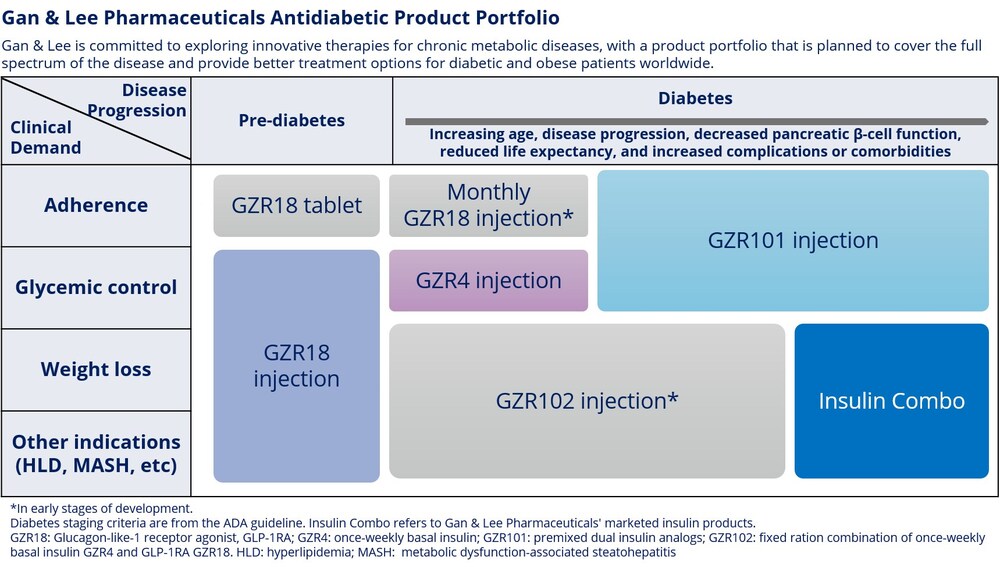
Clinical ResultPhase 2
22 Jun 2024
BEIJING and BRIDGEWATER, N.J., June 22, 2024 /PRNewswire/ -- Gan & Lee Pharmaceuticals (Gan & Lee, Shanghai Stock Exchange: 603087) announced the results of the Phase 1b/2a clinical study of the Company's independently developed glucagon-like peptide-1 (GLP-1) receptor agonist, GZR18 Injection, in an obese/overweight population in China, along with the results of two other innovative insulins' preclinical studies in poster presentations at the American Diabetes Association's(ADA's)84th Scientific Sessions.
This randomized, double-blind, placebo-controlled, dose-escalation Phase 1b/2a clinical study evaluated the safety, tolerability, pharmacokinetics and efficacy of GZR18 Injection in Chinese subjects with obesity/overweight after multiple administration on a once-weekly (QW) or bi-weekly (Q2W) dosing interval. A total of 36 obese participants were enrolled in the study and randomized in a 3:1 ratio to receive a dose titration of 1.5 mg to 30 mg of GZR18 Injection or a matching placebo for a total of 35 weeks.
The study results demonstrated a superior efficacy of GZR18 Injection than placebo for weight reduction in Chinese obese subjects. After 35 weeks of treatment, the mean weight change from baseline in the GZR18 QW group was -16.5 kg (95% CI: -19.9 kg, -13.1 kg); the placebo-adjusted mean percent weight change from baseline was -18.6% (95% CI: -25.5%, -11.6%). Although it was not a head-to-head study, when compared to the published data on weight reduction of similar products currently available on the market, GZR18's weight-reducing ability outperformed Semaglutide and dual-incretin receptor targeted Tirzepatide in similar study duration. Meanwhile, the mean weight change from baseline in the GZR18 Q2W group was -11.3 kg (95% CI: -15.4 kg, -7.2 kg); the placebo-adjusted mean percent weight change from baseline was -13.5% (95% CI: -21.0%, -6.0%).
In addition, the percentage of participants achieving weight reductions of ≥5%, 10%, and 15% from baseline were 100.0%, 90.0%, and 80.0%, respectively, in the GZR18 QW group, and the percentage of participants achieving weight reductions of ≥5%, 10%, and 15% from baseline were 71.4%, 71.4%, and 42.9%, respectively, in the GZR18 Q2W group. No participant in the placebo group achieved a weight reduction of 5% and above.
In terms of safety, GZR18 Injection was well tolerated in obese participants. The most commonly reported adverse events (AE) during treatment were gastrointestinal related AEs, and all were mild to moderate in severity. This is consistent with the incretin-based therapies approved for the treatment of obesity and overweight and occurred mainly in the early dose-escalation period. There were no serious hypoglycemic events in this study and no serious adverse events related to the investigational drug.
Gan & Lee also announced that a multi-center, placebo-controlled, randomized, double-blind, 30-week Phase 2 clinical study evaluating the efficacy and safety of GZR18 Injection in Chinese adults with obesity and overweight is in progress. A total of 338 adults with obesity or overweight were enrolled in this study, and the study explores a broader dose range and frequency of administration. The main body of the Phase 2 has now been completed, and the priliminary study data further support the results of the reported Phase 1b/2a obese/overweight study, particularly the positive results achieved with bi-weekly dosing frequency.
"We are very excited about the clinical results of the GZR18 program to the present day." Dr. Gan Zhong-ru, Founder of Gan & Lee, commented. "Our unique molecular design delays the onset of drug action and attenuates the peak effect, thereby improving drug tolerability and achieving smooth and sustained weight loss in a stepwise manner. Moreover, GZR18 has a longer duration of action, which is expected to be administered once every two weeks. Meanwhile, we hope that the clinical results of GZR18 will provide more evidence to reveal the mechanism of action of different targets of Incretins and Glucagon."
In addition, Gan & Lee announced the results of preclinical trials of the company's investigational products: GZR4, a once-weekly insulin analog, and GZR101, a premixed dual insulin analog, at the ADA's 84th Scientific Sessions:
Once-weekly Insulin Analog GZR4
GZR4 is a novel ultra-long-acting basal insulin analog designed for once-weekly administration. Results from preclinical studies have shown that GZR4 has a significantly higher affinity for human serum albumin (HSA) and a significantly lower affinity for the insulin receptor than insulin Icodec, another once-weekly insulin analog. Moreover, unlike insulin Icodec, GZR4 maintains its activity in activating the insulin receptor after binding to albumin. In the studies using animal models of diabetes, the glucose-lowering effect of GZR4 was observed to be 2-3 times greater than that of insulin Icodec. Based on the preclinical results, GZR4 is expected to be the fourth-generation basal insulin that can be administered once a week to achieve an effective glycemic control.
Premixed Insulin Analog GZR101
GZR101 injection is a premixed insulin analog made from a combination of ultra-long-acting basal insulin GZR33 injection and rapid-acting insulin aspart (Rapilin®). Different from traditional premixed insulin analogs, the duration of glucose-lowering effect of basal insulin component (GZR33) in GZR101 can last 72 hours, and there is no significant peak within 24 hours after reaching a steady state with multiple injections. When combined with insulin aspart (Rapilin®️) to make a premixed insulin analog, it can achieve smooth control of fasting and postprandial blood glucose throughout the day. In diabetic animal models, GZR101 is significantly superior than insulin degludec/insulin aspart (IDegAsp) in blood glucose reduction and safety. As a premixed insulin analog developed based on an advanced concept, GZR101 is expected to make an important contribution to the control of blood glucose and the reduction of the risk of hypoglycemia in patients with diabetes globally.
Conclusions and Future Directions
The ADA's 84th scientific sessions highlighted Gan & Lee Pharmaceuticals' leadership in developing next-generation diabetes and obesity treatments. With these latest preclinical and clinical results, the Company will continue to advance the development of innovative therapeutics for diabetes. While ongoing studies and upcoming trials will further support the positive influence of these innovative medicines on public health issues related to diabetes and obesity.
Forward-looking Statements
Forward-looking statements are based on our expectations and assumptions as of the date of the statements. Actual results may differ materially from those expressed in these forward-looking statements due to a variety of factors, and we can give no assurance that such results will be achieved in the future. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
References
:
American Diabetes Association.
ADA's 84th Scientific Sessions.
About Gan & Lee
Gan & Lee Pharmaceuticals developed the first Chinese domestic insulin analog. Currently, Gan & Lee has six core insulin products, including five insulin analog varieties: long-acting glargine injection (Basalin®), fast-acting lispro injection (Prandilin™), fast-acting aspart injection (Rapilin®), mixed protamine zinc lispro injection (25R) (Prandilin™25), aspart 30 injection (Rapilin®30), and one human insulin injection - mixed protamine human insulin injection (30R) (Similin®30). The company has two approved medical devices in China, namely reusable insulin injection pen (GanleePen), and disposable pen needle (GanleeFine®).
In China's 2024 National Insulin-Specific Centralized Procurement, Gan & Lee Pharmaceuticals ranked second overall and first among domestic companies in terms of procurement demand for insulin analogs. The company is also making strides in international markets, with the disposable pen needle (GanleeFine®) approved by the US Food and Drug Administration (FDA) in 2020 and received GMP inspection approval from the European Medicines Agency (EMA) in 2024. These achievements significantly boost Gan & Lee's competitiveness in both international and domestic markets.
In the future, Gan & Lee will strive for comprehensive coverage in diabetes treatment. Moving forward with its mission to become a world-class pharmaceutical company, Gan & Lee will also actively develop new chemical entities and biological drugs, focusing on treatments for metabolic diseases, cardiovascular diseases, and other therapeutic areas.
Further Information:
[email protected] (Media)
[email protected] (Business Development)
SOURCE Gan & Lee Pharmaceuticals
Clinical ResultPhase 2Drug Approval
100 Deals associated with Insulin aspart biosimilar(Gan & Lee Pharmaceuticals Co., Ltd.)
Login to view more data
R&D Status
10 top approved records. to view more data
Login
| Indication | Country/Location | Organization | Date |
|---|---|---|---|
| Diabetes Mellitus | Kazakhstan | 26 Aug 2022 | |
| Diabetes Mellitus, Type 1 | China | 05 Jun 2020 | |
| Diabetes Mellitus, Type 2 | China | 05 Jun 2020 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
No Data | |||||||
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or

Deal
Boost your decision using our deal data.
login
or

Core Patent
Boost your research with our Core Patent data.
login
or

Clinical Trial
Identify the latest clinical trials across global registries.
login
or

Approval
Accelerate your research with the latest regulatory approval information.
login
or

Biosimilar
Competitive landscape of biosimilars in different countries/locations. Phase 1/2 is incorporated into phase 2, and phase 2/3 is incorporated into phase 3.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free
