Request Demo
Last update 24 May 2025
Bavunalimab
Last update 24 May 2025
Overview
Basic Info
Drug Type Bispecific antibody |
Synonyms Anti-CTLA-4/anti-LAG-3 bispecific monoclonal antibody, CTLA-4xLAG-3 bispecific antibody, Pavunalimab + [3] |
Target |
Action inhibitors |
Mechanism CTLA4 inhibitors(Cytotoxic T-Lymphocyte-Associated Antigen 4 inhibitors), LAG3 inhibitors(Lymphocyte activation gene 3 protein inhibitors) |
Therapeutic Areas |
Active Indication- |
Inactive Indication |
Originator Organization |
Active Organization- |
Inactive Organization |
License Organization- |
Drug Highest PhaseDiscontinuedPhase 1 |
First Approval Date- |
Regulation- |
Login to view timeline
Structure/Sequence
Sequence Code 263405115

Source: *****
Sequence Code 263406856

Source: *****
Sequence Code 263407032

Source: *****
Related
2
Clinical Trials associated with BavunalimabNCT05695898
Phase Ib/II Study of XmAb23104 (PD1 X ICOS) and XmAb22841 (CTLA-4 X LAG3) Combination in Metastatic Melanoma Refractory to Prior Immune Checkpoint Inhibitor Therapy With and Without CNS Disease
This is a first-in-human, multi-center, multi-cohort, open-label, phase Ib/II study of XmAb22841 (CTLA-4 X LAG3) administered in combination with XmAb23104 (PD1 X ICOS) in participants with a histologically or cytologically confirmed diagnosis of an advanced/metastatic melanoma. XmAb22841 (CTLA-4 X LAG3) is a bi-specific antibody targeting two different T cell membrane proteins responsible for regulation of T cell activity. It offers potential immunologic and safety advantages over existing therapies. XmAb22841 (CTLA-4 X LAG3) is being evaluated in this clinical study designed to assess the safety, tolerability, PK, and PD of escalating doses of XmAb22841 (CTLA-4 X LAG3) administered in combination with XmAb23104 (PD1 X ICOS)
The study will be conducted through the University of California Melanoma Consortium (UCMC).
The study will be conducted through the University of California Melanoma Consortium (UCMC).
Start Date28 Feb 2023 |
Sponsor / Collaborator |
NCT03849469
A Phase 1 Multiple-Dose Study to Evaluate the Safety and Tolerability of XmAb®22841 Monotherapy and in Combination With Pembrolizumab in Subjects With Selected Advanced Solid Tumors (DUET-4)
This is a Phase 1, multiple dose, ascending-dose escalation study and expansion study designed to define a maximum tolerated dose and/or recommended dose of XmAb22841 monotherapy and in combination with pembrolizumab; to assess safety, tolerability, pharmacokinetics, immunogenicity, and anti-tumor activity of XmAb22841 monotherapy and in combination with pembrolizumab in subjects with select advanced solid tumors.
Start Date29 May 2019 |
Sponsor / Collaborator  Xencor, Inc. Xencor, Inc. [+1] |
100 Clinical Results associated with Bavunalimab
Login to view more data
100 Translational Medicine associated with Bavunalimab
Login to view more data
100 Patents (Medical) associated with Bavunalimab
Login to view more data
2
News (Medical) associated with Bavunalimab28 Sep 2022
NEW YORK, Sept. 28, 2022 /PRNewswire/ -- In the bispecific antibodies for cancer market,
emerging bispecific antibody generation platforms will be a key trend during the forecast period. For instance, Xencor is developing XmAb20717, XmAb22841, XmAb23104, XmAb24306, and XmAb14045, which are currently in the early phase of development, using the XmAb antibody engineering platform. In addition, Aptevo Therapeutics has developed APVO436 using its ADAPTIR platform for treating patients with acute myeloid leukemia. Therefore, such platforms will drive market growth during the forecast period.
Continue Reading
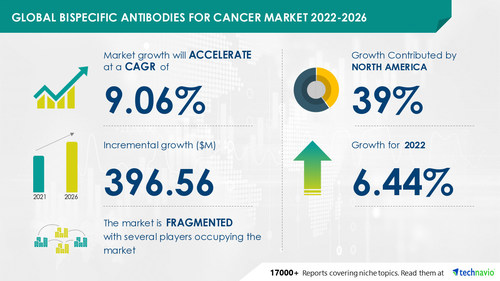
Technavio has announced its latest market research report titled Global Bispecific Antibodies for Cancer Market 2022-2026
The bispecific antibodies for cancer market size is expected to grow by USD 396.56 million from 2021 to 2026. In addition, the growth momentum of the market will accelerate at a CAGR of 9.06% during the forecast period, according to Technavio.
Our comprehensive report summary includes the market size and forecast along with
research methodology. The FREE sample report is available in PDF format
The increasing prevalence of cancer is driving the growth of the bispecific antibodies for cancer market growth. However, factors such as the high cost of drugs may challenge the market growth during the forecast period.
Bispecific Antibodies For Cancer Market: Type Landscape
By type, the market has been segmented into CD19 or CD3 and CD30 or CD16A. The
CD19 or CD3 segment will be the largest contributor to market growth during the forecast period. Bispecific antibodies have several advantages when compared to monoclonal antibodies. They can redirect specific immune cells to the site of the tumor cells to boost tumor killing. In addition, they can potentially increase the binding specificity by interacting with two different cell-surface antigens instead of one. They are also able to block two different pathways that exert unique pathogenesis simultaneously. Such advantages will drive the growth of the segment during the forecast period.
Bispecific Antibodies For Cancer Market: Geographic Landscape
Based on geography, the market has been segmented into North America, Europe, Asia, and Rest of World (ROW).
North America is expected to have significant growth during the forecast period. About 39% of the market's overall growth is expected to originate from this region. The US is the key country for the bispecific antibodies for the cancer market in North America. The growth of this region is attributed to factors such as the availability of reimbursement schemes. Moreover, this region is expected to grow at a faster rate than other regions.
Find out about the contribution of each region of the market. Buy Now to gain access to
country-specific information
Companies Covered
ABL Bio Inc.
AFFIMED N.V.
Akeso Inc.
Amgen Inc.
Aptevo Therapeutics Inc.
Astellas Pharma Inc.
Chugai Pharmaceutical Co. Ltd.
Eli Lilly and Co.
EPIMAB BIOTHERAPEUTICS INC.
F. Hoffmann La Roche Ltd.
F-STAR THERAPEUTICS INC.
Glenmark Pharmaceuticals Ltd.
Johnson and Johnson Inc.
Mereo BioPharma Group Plc
Merus N.V.
Pfizer Inc.
Regeneron Pharmaceuticals Inc.
TG Therapeutics Inc.
Xencor Inc.
Y mAbs Therapeutics Inc.
For learning about strategic initiatives used by vendors, as well as key news and the
latest developments, View our FREE PDF Sample Report Now
What our reports offer
Market share assessments for regional-level and country-level segments
Strategic recommendations for the new entrants
Covers market data for 2021, 2022, until 2026
Market trends (opportunities, drivers, threats, challenges, investment opportunities, and recommendations)
Strategic recommendations in key business segments as per the market approximations
Competitive landscaping highlighting the common trends
Company profiling with detailed strategies, recent developments, and financials
Supply chain trends that map the latest technological advances
Related Reports
Non-melanoma Skin Cancer Market by Type and Geography - Forecast and Analysis 2022-2026: The non-melanoma skin cancer market share is expected to increase by USD 180.97 million from 2021 to 2026.
Colorectal Cancer Therapeutics Market by Type and Geography - Forecast and Analysis 2022-2026: The colorectal cancer therapeutics market share is expected to increase by USD 2.45 bn from 2021 to 2026.
Browse Health Care Market Reports
Table of Contents
1 Executive Summary
1.1 Market overview
Exhibit 01: Executive Summary – Chart on Market Overview
Exhibit 02: Executive Summary – Data Table on Market Overview
Exhibit 03: Executive Summary – Chart on Global Market Characteristics
Exhibit 04: Executive Summary – Chart on Market by Geography
Exhibit 05: Executive Summary – Chart on Market Segmentation by Type
Exhibit 06: Executive Summary – Chart on Incremental Growth
Exhibit 07: Executive Summary – Data Table on Incremental Growth
Exhibit 08: Executive Summary – Chart on Vendor Market Positioning
2 Market Landscape
2.1 Market ecosystem
Exhibit 09: Parent market
Exhibit 10: Market Characteristics
3 Market Sizing
3.1 Market definition
Exhibit 11: Offerings of vendors included in the market definition
3.2 Market segment analysis
Exhibit 12: Market segments
3.3 Market size 2021
3.4 Market outlook: Forecast for 2021-2026
Exhibit 13: Chart on Global - Market size and forecast 2021-2026 ($ million)
Exhibit 14: Data Table on Global - Market size and forecast 2021-2026 ($ million)
Exhibit 15: Chart on Global Market: Year-over-year growth 2021-2026 (%)
Exhibit 16: Data Table on Global Market: Year-over-year growth 2021-2026 (%)
4 Five Forces Analysis
4.1 Five forces summary
Exhibit 17: Five forces analysis - Comparison between 2021 and 2026
4.2 Bargaining power of buyers
Exhibit 18: Bargaining power of buyers – Impact of key factors in 2021 and 2026
4.3 Bargaining power of suppliers
Exhibit 19: Bargaining power of suppliers – Impact of key factors in 2021 and 2026
4.4 Threat of new entrants
Exhibit 20: Threat of new entrants – Impact of key factors in 2021 and 2026
4.5 Threat of substitutes
Exhibit 21: Threat of substitutes – Impact of key factors in 2021 and 2026
4.6 Threat of rivalry
Exhibit 22: Threat of rivalry – Impact of key factors in 2021 and 2026
4.7 Market condition
Exhibit 23: Chart on Market condition - Five forces 2021 and 2026
5 Market Segmentation by Type
5.1 Market segments
Exhibit 24: Chart on Type - Market share 2021-2026 (%)
Exhibit 25: Data Table on Type - Market share 2021-2026 (%)
5.2 Comparison by Type
Exhibit 26: Chart on Comparison by Type
Exhibit 27: Data Table on Comparison by Type
5.3 CD19 or CD3 - Market size and forecast 2021-2026
Exhibit 28: Chart on CD19 or CD3 - Market size and forecast 2021-2026 ($ million)
Exhibit 29: Data Table on CD19 or CD3 - Market size and forecast 2021-2026 ($ million)
Exhibit 30: Chart on CD19 or CD3 - Year-over-year growth 2021-2026 (%)
Exhibit 31: Data Table on CD19 or CD3 - Year-over-year growth 2021-2026 (%)
5.4 CD30 or CD16A - Market size and forecast 2021-2026
Exhibit 32: Chart on CD30 or CD16A - Market size and forecast 2021-2026 ($ million)
Exhibit 33: Data Table on CD30 or CD16A - Market size and forecast 2021-2026 ($ million)
Exhibit 34: Chart on CD30 or CD16A - Year-over-year growth 2021-2026 (%)
Exhibit 35: Data Table on CD30 or CD16A - Year-over-year growth 2021-2026 (%)
5.5 Market opportunity by Type
Exhibit 36: Market opportunity by Type ($ million)
6 Customer Landscape
6.1 Customer landscape overview
Exhibit 37: Analysis of price sensitivity, lifecycle, customer purchase basket, adoption rates, and purchase criteria
7 Geographic Landscape
7.1 Geographic segmentation
Exhibit 38: Chart on Market share by geography 2021-2026 (%)
Exhibit 39: Data Table on Market share by geography 2021-2026 (%)
7.2 Geographic comparison
Exhibit 40: Chart on Geographic comparison
Exhibit 41: Data Table on Geographic comparison
7.3 North America - Market size and forecast 2021-2026
Exhibit 42: Chart on North America - Market size and forecast 2021-2026 ($ million)
Exhibit 43: Data Table on North America - Market size and forecast 2021-2026 ($ million)
Exhibit 44: Chart on North America - Year-over-year growth 2021-2026 (%)
Exhibit 45: Data Table on North America - Year-over-year growth 2021-2026 (%)
7.4 Europe - Market size and forecast 2021-2026
Exhibit 46: Chart on Europe - Market size and forecast 2021-2026 ($ million)
Exhibit 47: Data Table on Europe - Market size and forecast 2021-2026 ($ million)
Exhibit 48: Chart on Europe - Year-over-year growth 2021-2026 (%)
Exhibit 49: Data Table on Europe - Year-over-year growth 2021-2026 (%)
7.5 Asia - Market size and forecast 2021-2026
Exhibit 50: Chart on Asia - Market size and forecast 2021-2026 ($ million)
Exhibit 51: Data Table on Asia - Market size and forecast 2021-2026 ($ million)
Exhibit 52: Chart on Asia - Year-over-year growth 2021-2026 (%)
Exhibit 53: Data Table on Asia - Year-over-year growth 2021-2026 (%)
7.6 Rest of World (ROW) - Market size and forecast 2021-2026
Exhibit 54: Chart on Rest of World (ROW) - Market size and forecast 2021-2026 ($ million)
Exhibit 55: Data Table on Rest of World (ROW) - Market size and forecast 2021-2026 ($ million)
Exhibit 56: Chart on Rest of World (ROW) - Year-over-year growth 2021-2026 (%)
Exhibit 57: Data Table on Rest of World (ROW) - Year-over-year growth 2021-2026 (%)
7.7 US - Market size and forecast 2021-2026
Exhibit 58: Chart on US - Market size and forecast 2021-2026 ($ million)
Exhibit 59: Data Table on US - Market size and forecast 2021-2026 ($ million)
Exhibit 60: Chart on US - Year-over-year growth 2021-2026 (%)
Exhibit 61: Data Table on US - Year-over-year growth 2021-2026 (%)
7.8 Ireland - Market size and forecast 2021-2026
Exhibit 62: Chart on Ireland - Market size and forecast 2021-2026 ($ million)
Exhibit 63: Data Table on Ireland - Market size and forecast 2021-2026 ($ million)
Exhibit 64: Chart on Ireland - Year-over-year growth 2021-2026 (%)
Exhibit 65: Data Table on Ireland - Year-over-year growth 2021-2026 (%)
7.9 China - Market size and forecast 2021-2026
Exhibit 66: Chart on China - Market size and forecast 2021-2026 ($ million)
Exhibit 67: Data Table on China - Market size and forecast 2021-2026 ($ million)
Exhibit 68: Chart on China - Year-over-year growth 2021-2026 (%)
Exhibit 69: Data Table on China - Year-over-year growth 2021-2026 (%)
7.10 Hungary - Market size and forecast 2021-2026
Exhibit 70: Chart on Hungary - Market size and forecast 2021-2026 ($ million)
Exhibit 71: Data Table on Hungary - Market size and forecast 2021-2026 ($ million)
Exhibit 72: Chart on Hungary - Year-over-year growth 2021-2026 (%)
Exhibit 73: Data Table on Hungary - Year-over-year growth 2021-2026 (%)
7.11 India - Market size and forecast 2021-2026
Exhibit 74: Chart on India - Market size and forecast 2021-2026 ($ million)
Exhibit 75: Data Table on India - Market size and forecast 2021-2026 ($ million)
Exhibit 76: Chart on India - Year-over-year growth 2021-2026 (%)
Exhibit 77: Data Table on India - Year-over-year growth 2021-2026 (%)
7.12 Market opportunity by geography
Exhibit 78: Market opportunity by geography ($ million)
8 Drivers, Challenges, and Trends
8.1 Market drivers
8.2 Market challenges
8.3 Impact of drivers and challenges
Exhibit 79: Impact of drivers and challenges in 2021 and 2026
8.4 Market trends
9 Vendor Landscape
9.1 Overview
9.2 Vendor landscape
Exhibit 80: Overview on Criticality of inputs and Factors of differentiation
9.3 Landscape disruption
Exhibit 81: Overview on factors of disruption
9.4 Industry risks
Exhibit 82: Impact of key risks on business
10 Vendor Analysis
10.1 Vendors covered
Exhibit 83: Vendors covered
10.2 Market positioning of vendors
Exhibit 84: Matrix on vendor position and classification
10.3 ABL Bio Inc.
Exhibit 85: ABL Bio Inc. - Overview
Exhibit 86: ABL Bio Inc. - Product / Service
Exhibit 87: ABL Bio Inc. - Key offerings
10.4 Amgen Inc.
Exhibit 88: Amgen Inc. - Overview
Exhibit 89: Amgen Inc. - Product / Service
Exhibit 90: Amgen Inc. - Key offerings
10.5 Astellas Pharma Inc.
Exhibit 91: Astellas Pharma Inc. - Overview
Exhibit 92: Astellas Pharma Inc. - Product / Service
Exhibit 93: Astellas Pharma Inc. - Key news
Exhibit 94: Astellas Pharma Inc. - Key offerings
10.6 Eli Lilly and Co.
Exhibit 95: Eli Lilly and Co. - Overview
Exhibit 96: Eli Lilly and Co. - Business segments
Exhibit 97: Eli Lilly and Co. - Key offerings
Exhibit 98: Eli Lilly and Co. - Segment focus
10.7 F. Hoffmann La Roche Ltd.
Exhibit 99: F. Hoffmann La Roche Ltd. - Overview
Exhibit 100: F. Hoffmann La Roche Ltd. - Business segments
Exhibit 101: F. Hoffmann La Roche Ltd. - Key news
Exhibit 102: F. Hoffmann La Roche Ltd. - Key offerings
Exhibit 103: F. Hoffmann La Roche Ltd. - Segment focus
10.8 Johnson and Johnson Inc.
Exhibit 104: Johnson and Johnson Inc. - Overview
Exhibit 105: Johnson and Johnson Inc. - Business segments
Exhibit 106: Johnson and Johnson Inc. - Key news
Exhibit 107: Johnson and Johnson Inc. - Key offerings
Exhibit 108: Johnson and Johnson Inc. - Segment focus
10.9 Merus N.V.
Exhibit 109: Merus N.V. - Overview
Exhibit 110: Merus N.V. - Key offerings
10.10 Pfizer Inc.
Exhibit 111: Pfizer Inc. - Overview
Exhibit 112: Pfizer Inc. - Product / Service
Exhibit 113: Pfizer Inc. - Key news
Exhibit 114: Pfizer Inc. - Key offerings
10.11 Regeneron Pharmaceuticals Inc.
Exhibit 115: Regeneron Pharmaceuticals Inc. - Overview
Exhibit 116: Regeneron Pharmaceuticals Inc. - Product / Service
Exhibit 117: Regeneron Pharmaceuticals Inc. - Key offerings
10.12 TG Therapeutics Inc.
Exhibit 118: TG Therapeutics Inc. - Overview
Exhibit 119: TG Therapeutics Inc. - Product / Service
Exhibit 120: TG Therapeutics Inc. - Key offerings
11 Appendix
11.1 Scope of the report
11.2 Inclusions and exclusions checklist
Exhibit 121: Inclusions checklist
Exhibit 122: Exclusions checklist
11.3 Currency conversion rates for US$
Exhibit 123: Currency conversion rates for US$
11.4 Research methodology
Exhibit 124: Research methodology
Exhibit 125: Validation techniques employed for market sizing
Exhibit 126: Information sources
11.5 List of abbreviations
Exhibit 127: List of abbreviations
About Us
Technavio is a leading global technology research and advisory company. Their research and analysis focus on emerging market trends and provide actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions. With over 500 specialized analysts, Technavio's report library consists of more than 17,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio's comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios.
Contact
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
Email: [email protected]
Website:
SOURCE Technavio
CollaborateAntibody
14 Jun 2022
NEW YORK & BOSTON & WILMINGTON, Del.--(BUSINESS WIRE)-- Pfizer Inc. (NASDAQ:PFE), MorphoSys USA, Inc., a fully owned subsidiary of MorphoSys AG (FSE: MOR; NASDAQ:MOR), and Incyte Corporation (NASDAQ:INCY) today announced a clinical trial collaboration and supply agreement to investigate the immunotherapeutic combination of Pfizer’s TTI-622, a novel SIRPα-Fc fusion protein, and Monjuvi® (tafasitamab-cxix) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who are not eligible for autologous stem cell transplantation (ASCT).
“TTI-622 blocks the signal-regulatory protein (SIRP)α–CD47 axis, which is a key checkpoint expected to become an important backbone immunotherapy across multiple tumors, especially hematological cancers,” said Chris Boshoff, M.D., Ph.D., Chief Development Officer, Oncology, Pfizer Global Product Development. “The early results for TTI-622 in late-line advanced lymphoid malignancies reflect the potential for class-leading monotherapy activity, and preclinical evidence with a diverse set of therapeutic agents provide a strong rationale for testing combination therapies. We are pleased to collaborate with MorphoSys and Incyte, generating additional evidence on the potential of TTI-622 to improve outcomes for patients with DLBCL.”
‟Monjuvi in combination with lenalidomide is an important treatment option for patients with relapsed or refractory diffuse large B-cell lymphoma, and its mechanism of action, efficacy and safety pro it an attractive combination partner,” said Malte Peters, M.D., MorphoSys Chief Research and Development Officer. “We believe that the addition of novel immunotherapies, such as the investigational anti-CD47 blocking agent TTI-622, to the backbone of Monjuvi plus lenalidomide have the potential to provide new meaningful combination treatment options for patients with relapsed or refractory diffuse large B-cell lymphoma.”
‟This collaboration has the potential to advance patient care in an area where there continues to be significant unmet medical need,” said Lance Leopold, M.D., Group Vice President, Clinical Development Hematology and Oncology at Incyte. “We are proud to support this research effort to evaluate the potential of a new chemotherapy-free combination for these patients.”
Pfizer’s TTI-622 is currently in Phase 1b/2 development across several indications, with a focus on hematological malignancies. CD47 is an innate immune checkpoint that binds SIRPα and delivers a "don’t eat me" signal to suppress macrophage phagocytosis. Overexpression of CD47 in solid and hematological malignancies, including in DLBCL, is associated with poor prognosis.
Monjuvi (marketed ex-U.S. as Minjuvi®), a CD19-directed immunotherapy, in combination with lenalidomide is a treatment for adult patients with relapsed or refractory DLBCL not otherwise specified, and who are not eligible for ASCT. In this indication, accelerated or conditional approvals were granted by the U.S. Food and Drug Administration, the European Medicines Agency and other regulatory authorities. Monjuvi is being co-commercialized by MorphoSys and Incyte in the United States. Incyte has exclusive commercialization rights outside the United States.
Preclinical data by Morphosys have shown a strong synergy of Monjuvi and anti-CD47 antibodies in in vitro and in vivo lymphoma models, providing scientific rationale for investigating this combination in clinical trials. This preclinical data was presented at the 62nd American Society of Hematology (ASH) Annual Meeting & Exposition in 2020.
Under the terms of the agreement, Pfizer will initiate a multicenter, international Phase 1b/2 study of TTI-622 with Monjuvi and lenalidomide for patients with relapsed or refractory DLBCL who are not eligible for ASCT. MorphoSys and Incyte will provide Monjuvi for the study, which will be sponsored and funded by Pfizer and is planned to be conducted in North America, Europe and Asia-Pacific.
The collaboration is effective immediately upon the execution of the agreement.
About Tafasitamab
Tafasitamab is a humanized Fc-modified CD19 targeting immunotherapy. In 2010, MorphoSys licensed exclusive worldwide rights to develop and commercialize tafasitamab from Xencor, Inc. Tafasitamab incorporates an XmAb® engineered Fc domain, which mediates B-cell lysis through apoptosis and immune effector mechanism including Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC) and Antibody-Dependent Cellular Phagocytosis (ADCP).
In the United States, Monjuvi® (tafasitamab-cxix) is approved by the U.S. Food and Drug Administration in combination with lenalidomide for the treatment of adult patients with relapsed or refractory DLBCL not otherwise specified, including DLBCL arising from low grade lymphoma, and who are not eligible for autologous stem cell transplant (ASCT). This indication is approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
In Europe, Minjuvi® (tafasitamab) received conditional marketing authorization in combination with lenalidomide, followed by Minjuvi monotherapy, for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who are not eligible for autologous stem cell transplant (ASCT).
Tafasitamab is being clinically investigated as a therapeutic option in B-cell malignancies in several ongoing combination trials.
Monjuvi® and Minjuvi® are registered trademarks of MorphoSys AG. Tafasitamab is co-marketed by Incyte and MorphoSys under the brand name MONJUVI® in the U.S., and marketed by Incyte under the brand name Minjuvi® in Europe, the UK and Canada.
XmAb® is a registered trademark of Xencor, Inc.
About Pfizer Oncology
At Pfizer Oncology, we are committed to advancing medicines wherever we believe we can make a meaningful difference in the lives of people living with cancer. Today, we have an industry-leading portfolio of 24 approved innovative cancer medicines and biosimilars across more than 30 indications, including breast, genitourinary, colorectal, blood and lung cancers, as well as melanoma.
About Pfizer: Breakthroughs That Change Patients’ Lives
At Pfizer, we apply science and our global resources to bring therapies to people that extend and significantly improve their lives. We strive to set the standard for quality, safety and value in the discovery, development and manufacture of health care products, including innovative medicines and vaccines. Every day, Pfizer colleagues work across developed and emerging markets to advance wellness, prevention, treatments and cures that challenge the most feared diseases of our time. Consistent with our responsibility as one of the world’s premier innovative biopharmaceutical companies, we collaborate with health care providers, governments and local communities to support and expand access to reliable, affordable health care around the world. For more than 170 years, we have worked to make a difference for all who rely on us. We routinely post information that may be important to investors on our website at www.Pfizer.com. In addition, to learn more, please visit us on www.Pfizer.com and follow us on Twitter at @Pfizer and @Pfizer News, LinkedIn,YouTube and like us on Facebook at Facebook.com/Pfizer.
About MorphoSys
At MorphoSys, we are driven by our mission: More life for people with cancer. As a global commercial-stage biopharmaceutical company, we use groundbreaking science and technologies to discover, develop, and deliver innovative cancer medicines to patients. MorphoSys is headquartered in Planegg, Germany, and has its U.S. operations anchored in Boston, Massachusetts. To learn more, visit us at and follow us on Twitter and LinkedIn.
About Incyte
Incyte is a Wilmington, Delaware-based, global biopharmaceutical company focused on finding solutions for serious unmet medical needs through the discovery, development and commercialization of proprietary therapeutics. For additional information on Incyte, please visit Incyte.com and follow @Incyte.
CollaborateAntibodyBiosimilarVaccineImmunotherapy
100 Deals associated with Bavunalimab
Login to view more data
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Brain Injuries | Phase 1 | United States | 28 Feb 2023 | |
| Metastatic melanoma | Phase 1 | United States | 28 Feb 2023 | |
| Adenocarcinoma of prostate | Phase 1 | United States | 29 May 2019 | |
| Advanced Malignant Solid Neoplasm | Phase 1 | United States | 29 May 2019 | |
| Anus Neoplasms | Phase 1 | United States | 29 May 2019 | |
| Endometrial Carcinoma | Phase 1 | United States | 29 May 2019 | |
| Fallopian Tube Carcinoma | Phase 1 | United States | 29 May 2019 | |
| Gastroesophageal junction adenocarcinoma | Phase 1 | United States | 29 May 2019 | |
| Hepatocellular Carcinoma | Phase 1 | United States | 29 May 2019 | |
| Intrahepatic Cholangiocarcinoma | Phase 1 | United States | 29 May 2019 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
No Data | |||||||
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or

Deal
Boost your decision using our deal data.
login
or

Core Patent
Boost your research with our Core Patent data.
login
or

Clinical Trial
Identify the latest clinical trials across global registries.
login
or

Approval
Accelerate your research with the latest regulatory approval information.
login
or

Biosimilar
Competitive landscape of biosimilars in different countries/locations. Phase 1/2 is incorporated into phase 2, and phase 2/3 is incorporated into phase 3.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free
