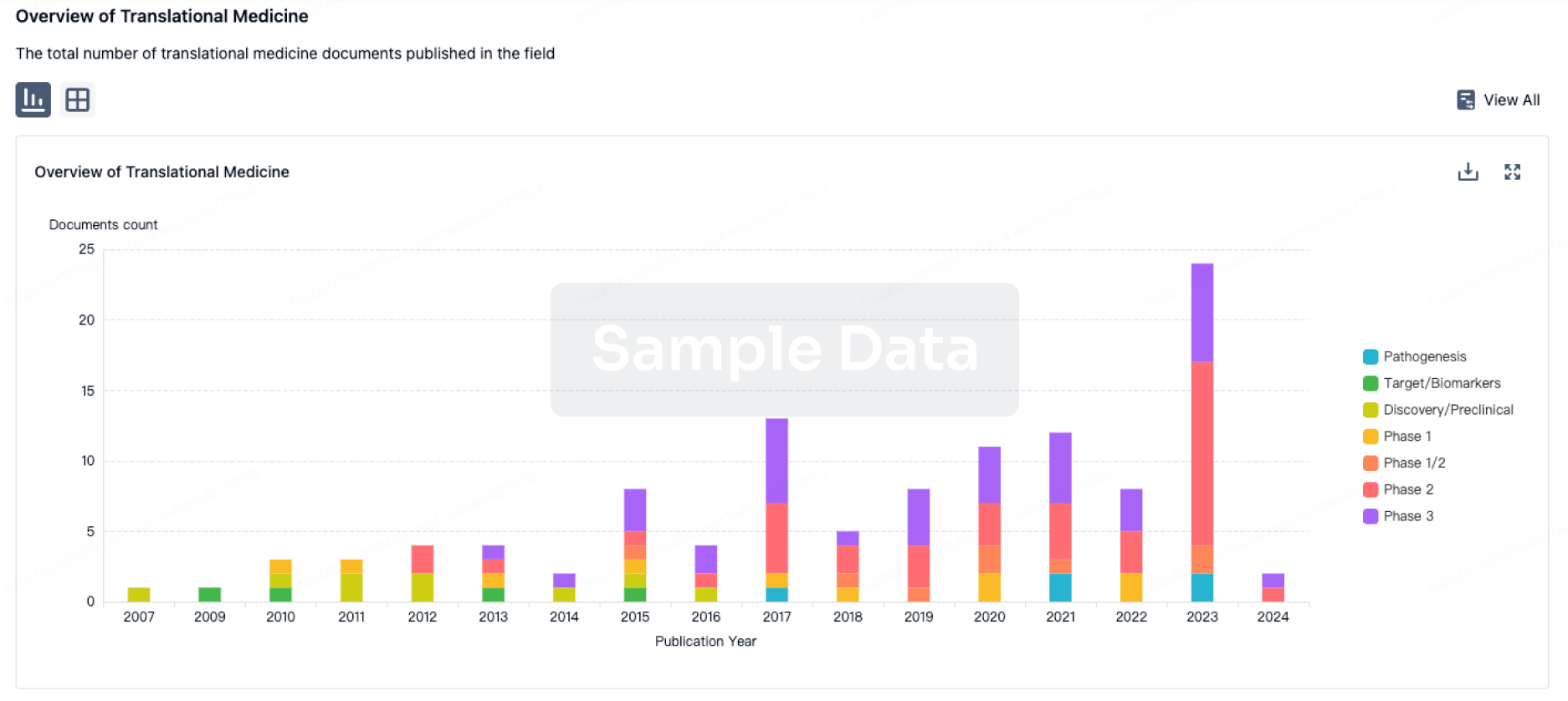
Record high core revenue, core operating profit and core net income for the fiscal year 2024 at ¥1,170.6 billion (+5.3%), ¥556.1 billion (+23.4%) and ¥397.1 billion (+19.0%) respectively (all changes year-on-year [YoY])
Steady progress in R&D activities of in-house projects for both early and late-stage development, with new investment activities
In early-stage development, NXT007 and AMY109 proceeded to Phase II clinical trials, and several projects started clinical trials. BRY10, a project applied with Chugai’s AI-assisted drug discovery technology, entered the clinical trial stage PiaSky, Alecensa, and NEMLUVIO received global approval In addition, Chugai Venture Fund made three investments
Planned 2024 year-end dividends are ¥57 per share (annual dividends for the fiscal year: ¥98 per share)
Core revenue and core operating profit in 2025 are expected to reach record highs of ¥1,190.0 billion (+1.7%, YoY) and ¥570.0 billion (+2.5%, YoY), respectively
Annual dividend forecast for 2025 is ¥250 per share, including 100th Anniversary Special Dividends of ¥150 per share
TOKYO, January 30, 2025 -- Chugai Pharmaceutical Co., Ltd. (TOKYO: 4519) announced its consolidated financial results for the fiscal year ended December 31, 2024, and forecasts for the fiscal year ending December 31, 2025.
“In 2024, Chugai marked record high revenue, operating profit, and net income. Despite the decrease in domestic sales due to impact of the completion of government supply of Ronapreve® for COVID-19, NHI drug price revisions and penetration of biosimilars, the greatly increased export of Hemlibra® to Roche and other factors contributed to the growth. In R&D, this year saw many achievements of global approvals for our in-house products. PiaSky® obtained initial approval for the treatment of paroxysmal nocturnal hemoglobinuria, and Alecensa® obtained approval for the additional indication of adjuvant treatment of non-small cell lung cancer in Japan, the United States, Europe and China. In addition, NEMLUVIO®, out-licensed to Galderma, obtained approval for the treatment of prurigo nodularis and atopic dermatitis in the United States. In early development of in-house projects, NXT007 and AMY109 proceeded to phase II clinical trials, and several projects including GYM329, DONQ52 and RAY121, have started new clinical trials. Furthermore, BRY10 marked the first project to enter the clinical development stage, which utilized Chugai’s proprietary AI-based antibody drug discovery support technology, MALEXA®. Chugai Venture Fund, which became fully operational in 2024, is implementing multiple investments that are expected to strengthen our company’s technology and drug discovery capabilities. 2025 marks the 100-year anniversary of the company’s founding. With the aspiration of ‘Creating drugs that benefit the world’ which has been consistently carried from the time of our founding, and by focusing on our unique science and technology capabilities and creating our unique innovation, Chugai will strive in the next 100 years, to continue to expand the benefit of medical community and human health around the world for the sake of patients,” said Dr. Osamu Okuda, Chugai’s President and CEO.
Chugai reported that revenue for the fiscal year ended December 2024 totaled ¥1,170.6 billion (+ ¥59.2 billion, +5.3%, YoY).
Domestic sales were ¥461.1 billion (- ¥96.9 billion, -17.4%, YoY). In the oncology field, although our new product Phesgo® performed well, products including our mainstay product Avastin® were affected by NHI drug price revisions and biosimilars. Also, sales of Perjeta® and Herceptin® fell along with the market penetration of Phesgo which includes the same active pharmaceutical ingredients, resulting in a decrease by 4.8% compared to the previous year. In the specialty field, sales decreased by 28.3% compared with previous year, mainly due to the completion of supply of Ronapreve to the government, despite that our new product Vabysmo® grew and our mainstay products Hemlibra and Actemra® performed well. Overseas sales were ¥536.8 billion (+ ¥120.3 billion, +28.9%, YoY), driven by a substantial increase in exports of Hemlibra to Roche. Other revenue increased by 26.2% mainly due to increase in one-time income, etc., in addition to the increase in income related to Hemlibra.
Cost to sales ratio improved by 8.4 percentage points year-on-year to 33.9%, mainly due to a change in the product mix. Research and development expenses amounted to ¥176.9 billion (+8.7%, YoY) due to investments into drug discovery and early development, and the progress of development projects. Selling, general and administration expenses were comparable to the previous year, amounting ¥102.2 billion (+0.2%, YoY). For other operating income (expense), an income of ¥2.7 billion was recorded, mainly due to the recognition of income from disposal of product rights. As a result, Core operating profit totaled ¥556.1 billion (+ ¥105.4 billion, +23.4%, YoY), which marked the first time exceeding ¥500.0 billion, and Core net income increased to ¥397.1 billion (+ ¥63.5 billion, +19.0%, YoY).
Reflecting the favorable results and based on our principles of “a stable allocation of profit” and “aiming for a consolidated dividend payout ratio of 45% on average in comparison with Core EPS,” year-end dividends for the fiscal year ended December 31, 2024 are planned to be ¥57 per share. As a result, the annual dividend per share will be ¥98 per share, and the Core dividend payout ratio is 40.6% (an average of 40.3% for the past five years).
Regarding research and development, Chugai made good progress in both early and late-stage development toward achieving TOP I 2030. Chugai made important progress in both in-house and in-licensed products, and in addition, through new investments, Chugai strengthened its efforts to create innovative solutions.
For in-house projects that will drive mid to long-term growth, NXT007 and AMY109, both applying Chugai’s proprietary antibody engineering technologies, have entered Phase II clinical trials. Additionally, RAY121 (for autoimmune diseases), BRY10 (for chronic diseases), GYM329 (for neurological disorders and obesity), and DONQ52 (for celiac disease) each have entered new clinical trials. In late-stage projects, PiaSky, a treatment for paroxysmal nocturnal hemoglobinuria, was launched in Japan and approved in the U.S., Europe, and China. Alecensa was approved for expanded indication as post-operative adjuvant therapy for ALK-positive non-small cell lung cancer in Japan, the U.S., Europe, China and Taiwan.
In-house products out-licensed to third parties excluding Roche also progressed steadily. NEMLUVIO (nemolizumab), being developed overseas by Galderma, has advanced significantly as a global product, having been approved for prurigo nodularis and moderate-to-severe atopic dermatitis in the U.S. and having been recommended for approval for the same indications by the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP). Avutometinib, out-licensed to Verastem Oncology, has been accepted for review of the New Drug Application (NDA) by the U.S. Food and Drug Administration (FDA) for accelerated approval in KRAS-mutant recurrent low-grade serous ovarian cancer and has been granted Priority Review.
As for projects in-licensed from Roche, Lunsumio® has received regulatory approval in Japan for relapsed or refractory follicular lymphoma. Elevidys (SRP-9001), Chugai’s first gene therapy product, has been filed with a regulatory application in Japan for Duchenne muscular dystrophy. Vabysmo and Evrysdi® received approvals for expanded indications, and Tecentriq® filed an application for expanded indication.
In 2024, to further accelerate our drug discovery engine through open innovation, Chugai Venture Fund, LLC made investments in Leal Therapeutics, HYKU Biosciences, and one other company, strengthening Chugai’s efforts in creating innovative solutions.
In 2025, Core revenues, Core operating profit, and Core net income are expected to be ¥1,190.0 billion (+ ¥19.4 billion, +1.7%, YoY), ¥570.0 billion (+ ¥13.9 billion, +2.5%, YoY), and ¥410.0 billion (+ ¥12.9 billion, +3.2%, YoY), resulting in an increase in both revenues and profits. Sales are expected to increase in Japan and overseas, totaling ¥1,018.0 billion (+ ¥20.1 billion, +2.0%, YoY). Domestic sales are expected to be ¥462.5 billion (+ ¥1.4 billion, +0.3%, YoY), including increase in volume of new product Phesgo, PiaSky and our mainstay products. Overseas sales are expected to be ¥555.5 billion (+ ¥18.7 billion, +3.5%, YoY) due to growth in sales of Hemlibra, Alecensa and NEMLUVIO, despite decrease in Actemra. Other revenues are expected to be ¥172.0 billion (- ¥0.7 billion, -0.4%, YoY). Royalty and profit-sharing income are forecasted to increase to ¥165.7 billion (+ ¥18.3 billion, +12.4%, YoY), due to an increase in income related to Hemlibra, despite a decrease in income related to Actemra. On the other hand, other operating income is expected to decrease to ¥6.3 billion (- ¥19.0 billion, -75.1%, YoY), due to a decrease in one time income.
For the following fiscal year ending December 31, 2025, Chugai expects annual dividends of ¥100 in regular dividends (interim dividends of ¥50 and year-end dividends of ¥50) and ¥150 as special dividends for the company's 100th anniversary (interim dividends of ¥75 and year-end dividends of ¥75) for a total of ¥250 per share. As a result, the Core dividend payout ratio for 2025 is expected to be 100.0% (54.1% on a five-year average basis).
[2024 full year results]
[Sales breakdown]
[Oncology field (Domestic) Top5-selling medicines]
+2.3%
[Specialty field (Domestic) Top5-selling medicines plus Ronapreve]
*Ronapreve has not been listed in the National Health Insurance (NHI) price list.
[2025 full year forecast]
[Progress in R&D activities from Oct 26th, 2024 to Jan 30th, 2025]
About Core results
Chugai discloses its results on a Core basis from 2013 in conjunction with its decision to apply IFRS. Core results are the results after adjusting Non-Core items to IFRS results. Chugai’s recognition of non-recurring items may differ from that of Roche due to the difference in the scale of operations, the scope of business and other factors. Core results are used by Chugai as an internal performance indicator, for explaining the underlying business performance both internally and externally, and as the basis for payment-by-results such as a return to shareholders.
Trademarks used or mentioned in this release are protected by law.
[PDF 1.3MB]
Contact:
For Media Chugai Pharmaceutical Co., Ltd. Media Relations Group, Corporate Communications Dept., Hideki Sato Tel: +81-3-3273-0881 E-mail: pr@chugai-pharm.co.jp
For Investors Chugai Pharmaceutical Co., Ltd. Investor Relations Group, Corporate Communications Dept., Takayuki Sakurai Tel: +81-3-3273-0554 E-mail: ir@chugai-pharm.co.jp
Back