A $1.7 Billion Global Opportunity for Hemoglobin A1C (HbA1C) Testing by 2026 - New Research from StrategyR
04 Jul 2022
AntibodyCollaborate
SAN FRANCISCO, July 4, 2022 /PRNewswire/ -- A new market study published by Global Industry Analysts Inc., (GIA) the premier market research company, today released its report titled "Hemoglobin A1C (HbA1C) Testing - Global Market Trajectory & Analytics". The report presents fresh perspectives on opportunities and challenges in a significantly transformed post COVID-19 marketplace.
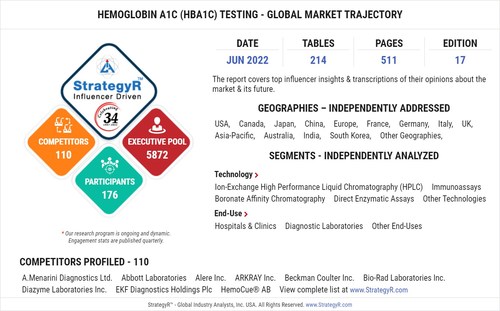
FACTS AT A GLANCE
What's New for 2022?
Preview
Source: PRNewswire
A $1.7 Billion Global Opportunity for Hemoglobin A1C (HbA1C) Testing by 2026 - New Research from StrategyR
Global competitiveness and key competitor percentage market shares
Market presence across multiple geographies - Strong/Active/Niche/Trivial
Online interactive peer-to-peer collaborative bespoke updates
Access to our digital archives and MarketGlass Research Platform
Complimentary updates for one year
Edition: 17;
Released: June 2022
Executive Pool: 5872
Companies: 110 - Players covered include A.Menarini Diagnostics Ltd.; Abbott Laboratories; Alere Inc.; ARKRAY Inc.; Beckman Coulter Inc.; Bio-Rad Laboratories Inc.; Diazyme Laboratories Inc.; EKF Diagnostics Holdings Plc; HemoCue® AB; OSANG Healthcare Co., Ltd; PTS Diagnostics; Roche Diagnostics; Siemens Healthineers; Tosoh Bioscience Inc.; Trinity Biotech plc and Others.
Coverage: All major geographies and key segments
Segments: Technology (Ion-Exchange High Performance Liquid Chromatography (HPLC), Immunoassays, Boronate Affinity Chromatography, Direct Enzymatic Assays, Other Technologies); End-Use (Hospitals & Clinics, Diagnostic Laboratories, Other End-Uses)
Geographies: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Complimentary Project Preview - This is an ongoing global program. Preview our research program before you make a purchase decision. We are offering a complimentary access to qualified executives driving strategy, business development, sales & marketing, and product management roles at featured companies. Previews provide deep insider access to business trends; competitive brands; domain expert profiles; and market data templates and much more. You may also build your own bespoke report using our MarketGlass™ Platform which offers thousands of data bytes without an obligation to purchase our report.
Preview Registry
ABSTRACT-
Amid the COVID-19 crisis, the global market for Hemoglobin A1C (HbA1C) Testing estimated at US$1.2 Billion in the year 2022, is projected to reach a revised size of US$1.7 Billion by 2026, growing at a CAGR of 8.4% over the analysis period. Ion-Exchange High Performance Liquid Chromatography (HPLC), one of the segments analyzed in the report, is projected to record a 7.5% CAGR, while growth in the Immunoassays segment is readjusted to a revised 8.1% CAGR. With diabetes reaching epidemic levels, there is strong potential for diabetes diagnostic and monitoring devices or tools including Hemoglobin A1c blood test. The adoption of HbA1c has gained momentum over the last decade as an essential assay for diabetes testing and monitoring. By measuring HbA1c levels in red blood cells, physicians gain access to information on patient's average concentration of glucose (sugar) level in the blood for the preceding 8-12 weeks. Given that red blood cells have a lifespan of 3 months (120 days) and HbA1c levels vary as new red blood cells are made, measuring HbA1c levels in a person's blood effectively gives an indication into the average blood glucose levels over the past two-to-three month period.
Hemoglobin A1c test is attracting the attention of the healthcare industry and patients alike by virtue of its numerous advantages. For example, HbA1c tests can be performed at any given time in a day and do not necessitate overnight fasting prior to the collection of blood sample, which is an essential requirement in case of traditional oral glucose tolerance test. Moreover, HbA1c testing needs to be performed on a quarterly-basis. Also, HbA1c test results provide physicians with information needed to develop intervention strategies and treatment plans to prevent diabetes-related complications. Healthcare professionals are increasingly choosing to prescribe HbA1c test for patients for tracking their blood glucose levels, as against blood glucose tests owing to the former's better reliability, thereby enhancing the overall test volume. Since A1c test determines the glucose levels in a patient over the prior 2-3 month period, it is considered to be an ideal and reliable option for tracking diabetes than the conventional blood glucose tests.
North America holds a commanding stake within the global hemoglobin A1c testing market and is predicted to retain its strong position over the coming years. The regional market is bolstered by increasing adoption of direct enzyme assay methods and technological advances related to the HPLC technology. The North American market is slated to gain from increasing acceptance of advanced chromatographic devices across hospitals, clinical settings and diagnostic laboratories. Led by Germany, Europe represents the second-leading market for hemoglobin A1c testing and is estimated to register a decent growth in the coming years. On the other hand, Asia-Pacific is forecast to post impressive gains and increase its market share due to rising incident of diabetes and the need for effective diagnostic techniques.
By technology type, global HbA1c testing devices market is categorized into ion-exchange HPLC, affinity binding chromatography, enzymatic assay, and turbidimetric inhibition immunoassay, among others. All types of techniques are utilized for estimating blood hemoglobin levels or for checking HbA1c concentrations in diabetic individuals. Affinity binding chromatography emerged as the largest technology segment, with segment growth fueled by the sustained increase in investments being made by medical device manufacturers in this technology. In boron affinity chromatography technique, HbA1c is known to bind with boronate affinity resin, thus separating from the non-HbA1c component. HbA1c gets released from boronic acid sites on the resin, following further elution with sorbitol, and the proportion of HbA1c release is measured using photometric absorbance techniques. Antigen molecule (HbA1c) interacts with specific antibody-coated latex beads (HbA1c specific) for enabling detection of concentrations of HbA1 and total hemoglobin in whole blood, a method that is termed as immunoassay or latex-enhanced immunoassay method. The interaction between HbA1c and Hba1c specific causes inhibition of agglutination. Due to inhibited agglutination, the light scattering reduces, thus leading to a reduction in absorbance. The calibrated curve of absorbance and related HbA1c concentration leads to showing of HbA1c percentages from absorbance readings. Other techniques used include capillary electrophoresis, proteomic analysis by mass spectrometry and direct enzymatic assay. Capillary electrophoresis technique is used for separating hemoglobin derivatives and hemoglobin variants. Mass spectrometry is utilized for detecting masses in globin chain in order to identify hemoglobin variant, HbA1c and total hemoglobin levels.
The ion-exchange high performance liquid chromatography (HPLC) segment claims the majority stake in the global hemoglobin A1c testing market and is projected to maintain its dominance in the coming years. The segment is estimated to exhibit a considerable growth rate due to several benefits of the technique over other options. Ion-exchange HPLC is fast, enhances ionic strength of the buffer, and identifies variants. The direct enzymatic assays segment is forecast to increase its market share due to continuous influx of new products. Enzymatic hemoglobin A1c testing is known for high reliability as enzymatic HbA1c assays remain unaffected by genetically- or chemically-modified hemoglobin variants. These benefits are anticipated to significantly drive global adoption of these assays.
High performance liquid chromatography (HPLC) testing for HbA1c has been perceived as the gold standard to monitor glucose control among diabetics since the separation of hemoglobin A1c from other types of hemoglobin using the chromatographic column in 1958. Despite the development and availability of alternative techniques over the following years, HPLC testing has successfully retained its value and is offering unique, significant advantages to hospitals, laboratories, medical professionals and patients. While the traditional format of HPLC was known for its time- and labor-intensive nature, continuing advances have addressed these issues and allowed seamless integration of HPLC testing for hemoglobin A1c on automated hematology platforms. These developments have enabled laboratories to offer the testing in an affordable manner. In the recent years, the hematology domain has witnessed the emergence of multi-discipline EDTA automation options. These approaches allow majority of tests on an EDTA tube by leveraging integration of standard hematology testing with specialized testing like HPLC for hemoglobin A1c testing. Though there are other approaches to determine HbA1c, there are concerns associated with chemical and genetic variants that lead to false negatives and false positives. However, the use of HPLC eliminates these concerns and allows reliable testing. There are different forms of HPLC including boronate affinity and ion-exchange methods that are commonly used for measuring HbA1c. In addition, the ion-exchange HPLC approach is capable of identifying different variants like C, D, E and S in the heterozygous state along with fetal Hb, LA1c and CHb.
More
MarketGlass™ Platform
Our MarketGlass™ Platform is a free full-stack knowledge center that is custom configurable to today`s busy business executive`s intelligence needs! This influencer driven interactive research platform is at the core of our primary research engagements and draws from unique perspectives of participating executives worldwide. Features include - enterprise-wide peer-to-peer collaborations; research program previews relevant to your company; 3.4 million domain expert profiles; competitive company profiles; interactive research modules; bespoke report generation; monitor market trends; competitive brands; create & publish blogs & podcasts using our primary and secondary content; track domain events worldwide; and much more. Client companies will have complete insider access to the project data stacks. Currently in use by 67,000+ domain experts worldwide.
Our platform is free for qualified executives and is accessible from our website www.StrategyR.com or via our just released mobile application on iOS or Android
About Global Industry Analysts, Inc. & StrategyR™
Global Industry Analysts, Inc., (www.strategyr.com) is a renowned market research publisher the world`s only influencer driven market research company. Proudly serving more than 42,000 clients from 36 countries, GIA is recognized for accurate forecasting of markets and industries for over 33 years.
CONTACTS:
Zak Ali
Director, Corporate Communications
Global Industry Analysts, Inc.
Phone: 1-408-528-9966
www.StrategyR.com
Email: [email protected]
LINKS
Join Our Expert Panel
https://www.strategyr.com/Panelist.asp
Connect With Us on LinkedIn
https://www.linkedin.com/company/global-industry-analysts-inc./
Follow Us on Twitter
https://twitter.com/marketbytes
[email protected]
SOURCE Global Industry Analysts, Inc.
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Organizations
Indications
Targets
Drugs
-Chat with Hiro
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.





