Clarity's theranostic prostate cancer trial advances to multi-dose phase
15 Mar 2024
Clinical ResultRadiation Therapy
Highlights
Cohort 3 of the theranostic SECuRE trial investigating 64Cu/67Cu-SAR-bisPSMA in metastatic castrate-resistant prostate cancer (mCRPC) has been completed in 6 participants who received therapy with 67Cu-SAR-bisPSMA at the highest single dose level of 12GBq.
No dose limiting toxicities (DLTs) have been reported in cohort 3 to date.
An overall safety review of all cohorts 1, 2 and 3 (4, 8 and 12GBq single dose, respectively) showed a favorable safety profile, with most adverse events (AEs) being mild or moderate.
The Safety Review Committee (SRC) has recommended that the trial progresses to cohort 4, a multi-dose phase, at the highest dose investigated in the study (12GBq).
Although the last participants in cohort 3 only completed dosing in January 2024, 60% of participants across all cohorts so far showed reductions in prostate-specific antigen (PSA) levels of greater than 35% from a single dose of 67Cu-SAR-bisPSMA. Twenty-seven percent of participants showed reductions in PSA levels of greater than 80%.
Among the participants from cohorts 2 and 3, almost 80% showed reductions in PSA levels of greater than 35% from a single dose of 67Cu-SAR-bisPSMA, and 44% showed reductions in PSA levels of greater than 80%.
Participants treated in the trial to date have received multiple lines of therapy prior to their recruitment into the study, including androgen deprivation therapy (ADT), androgen receptor pathway inhibition (ARPI) therapy, investigational agents, chemotherapy and other radioligand therapies such as alpha and beta-emitters (225Ac and 177Lu-based therapies, respectively).
Cohort 3 participants had the highest number of pre-treatments prior to entering the study across all cohorts, with most patients in this cohort receiving 5 or more lines of therapy. The number of lines of prior therapy in cohort 3 was almost double compared to cohort 2. Cohort 3 also had the highest pre-treatment median PSA across all cohorts (140.3 ng/ml, with a patient having the highest level in the cohort at 781.8 ng/ml).
Recruitment has opened for cohort 4, the first multi-dose cohort in the SECuRE trial, at clinical sites in the US. Participants in cohort 4 will receive multiple treatment cycles at the dose level of 12GBq of 67Cu-SAR-bisPSMA. All available patient slots for the first part of cohort 4 have been allocated and screening activities have commenced.
SYDNEY, March 15, 2024 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce the successful completion of cohort 3 and advancement to cohort 4, the first multi-dose cohort in the SECuRE trial.
Continue Reading
Preview
Source: PRNewswire
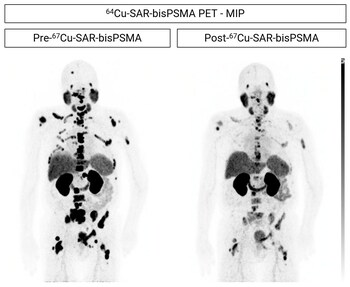
Fig 1. Participant from cohort 3 showing reduction in uptake of 64Cu-SAR-bisPSMA in prostate cancer lesions. The participant was treated with ADT, ARPI, chemotherapy and 2 investigational agents prior to enrolling in the SECuRE study (PSA 270.9 ng/ml at study entry). The participant received a single dose of 67Cu-SAR-bisPSMA (12GBq), which led to the reduction in uptake of 64Cu-SAR-bisPSMA in the lesions. PSA reduction: 92.3%.
Preview
Source: PRNewswire
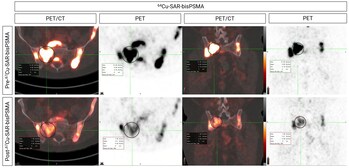
Fig 2. Participant from cohort 3 showing reduction in uptake of 64Cu-SAR-bisPSMA in pelvic bone lesions. mCRPC patient displaying the reduction in uptake of 64Cu-SAR-bisPSMA in pelvic bone lesions after receiving a single dose of 67Cu-SAR-bisPSMA (12GBq). The lesion highlighted in the circle shows a reduction in SUVmax from 31.6 to 8.0 (75% reduction, pre/post 67Cu-SAR-bisPSMA cycle, respectively). Left images: axial view. Right images: coronal view.
Preview
Source: PRNewswire
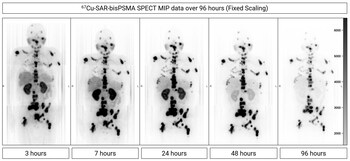
Fig 3. Dosimetry assessment in a participant from cohort 3 (12GBq). SPECT was performed at different timepoints (3, 7, 24, 48 and 96 hours post-injection of 67Cu-SAR-bisPSMA). Images show fast clearance from the kidneys, compared to prolonged retention of 67Cu-SAR-bisPSMA in lesions. MIP: maximum intensity projection.
Preview
Source: PRNewswire
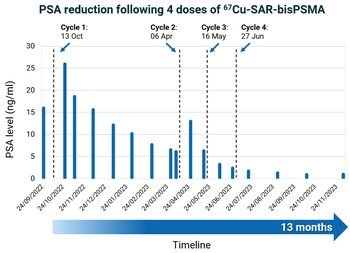
Graph 1. 76-year-old male with Gleason score 8 (4+4), mCRPC. Definitive radiation therapy in 2013. Previous treatments included ADT, abiraterone and enzalutamide. Thirteen months: time since the first dose of 67Cu-SAR-bisPSMA to most recent follow-up. EAP: Expanded Access Program. Dash lines: administration of 67Cu-SAR-bisPSMA. Data cut-off: 6 March 2024.
Preview
Source: PRNewswire
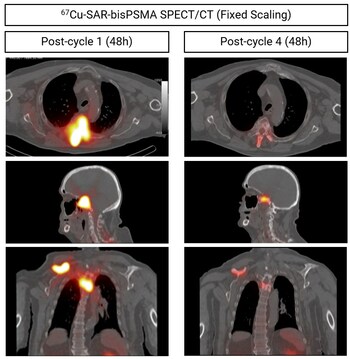
Fig 4. Same patient described in Graph 1 above (PSA reduction by 93.7%). Images show reduction in uptake of 67Cu-SAR-bisPSMA in multiple lesions (arrows) after 4 doses of 4GBq each using SPECT (images: SPECT/CT). Top images: axial view. Middle images: sagittal view. Bottom images: coronal view.
Preview
Source: PRNewswire
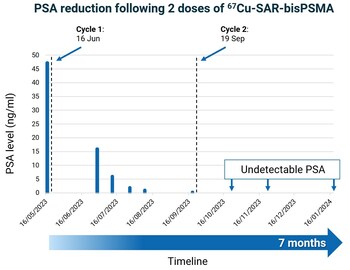
Graph 2. PSA reduction in mCRPC patient to undetectable levels following 2 doses of 67Cu-SAR-bisPSMA (8GBq). 74-year-old male with Gleason 9 (5+4) mCRPC (diagnosed in 2017). Previous treatments included ADT, Taxotere, abiraterone, enzalutamide and a clinical trial with a PARP inhibitor. Dash lines: administration of 67Cu-SAR-bisPSMA. Seven months: time since the first dose of 67Cu-SAR-bisPSMA to most recent follow-up. Lower level of PSA detection: 0.05 ng/ml. Data cut off: 6 March 2024.
Preview
Source: PRNewswire
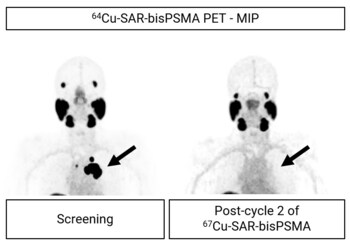
Figure 5. Same patient as shown in Graph 2 above. Image on the right show no lesion uptake of 64Cu-SAR-bisPSMA after two doses of 67Cu-SAR-bisPSMA (arrows). Images: maximum intensity projection.
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Targets
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.