Palleon Pharmaceuticals Reports Positive Phase 1 Results for E-602, a Revolutionary Glyco-Immune Checkpoint Inhibitor
21 Apr 2023
Clinical Study
PatSnap Synapse, a novel, AI Powered Drug Competitive Intelligence database. Access to drugs, clinical trials, patents, literature and news in one search.
Palleon Pharmaceuticals, a biopharmaceutical company focused on developing therapies for neoplasms, has presented initial results from the GLIMMER-01 clinical trial for E-602, the first-ever glyco-immune checkpoint inhibitor. This drug is an enzyme that stimulates T lymphocytes and is being developed as a potential treatment for neoplasms.
In the Phase 1 trial, E-602 was was found to be well tolerated across the entire dose range evaluated with no dose limiting toxicities. Having met pre-specified gating criteria based on safety and pharmacodynamic activity, Palleon announced plans to proceed with Phase 2 evaluation. Phase 2 studies will evaluate clinical activity of E-602 monotherapy in patients with lung cancer and melanoma.
E-602 is a promising drug being developed by Palleon Pharmaceuticals, Inc. It falls under the category of enzyme drugs and works by stimulating T lymphocytes. Its primary therapeutic focus is on neoplasms, and it is currently being tested in Phase 1/2 clinical trials for its effectiveness against neoplasms. Palleon Pharmaceuticals is the originator and active organization behind this innovative drug.
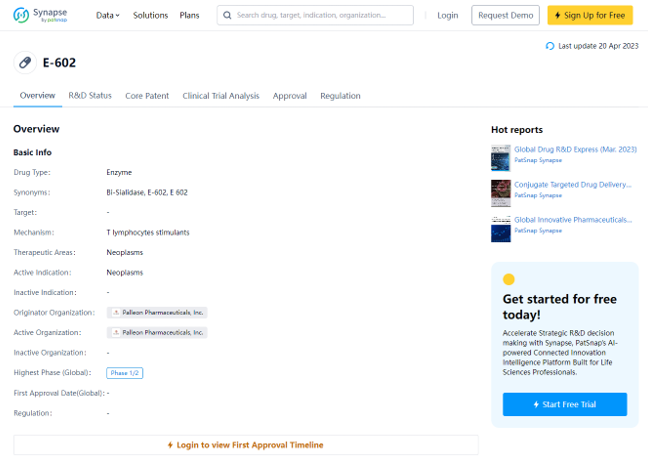
Preview
Source: SYNAPSE
Palleon Pharmaceuticals, Inc. was founded in 2016 and is headquartered in Massachusetts, United States. The company is listed on NASDAQ under the symbol MDGL and is focused on developing therapies for neoplasms using fusion protein and enzyme technologies. Their most
frequently developed targets include HER2, PDL1, and SIGLEC1.
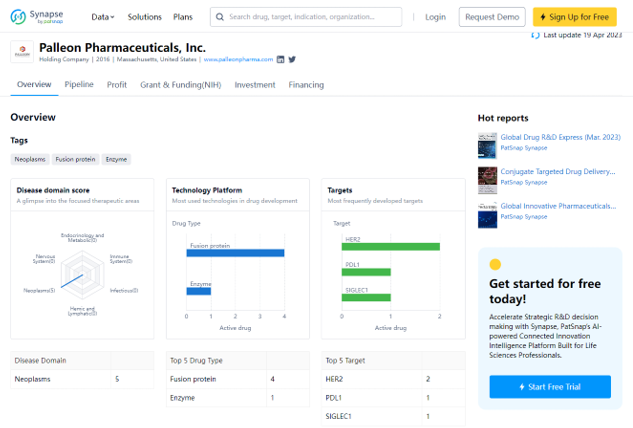
Preview
Source: SYNAPSE
In summary, the initial results from the GLIMMER-01 trial for E-602, a glyco-immune checkpoint inhibitor developed by Palleon Pharmaceuticals, show promise as a potential treatment for neoplasms. The company's focus on developing therapies for neoplasms using fusion protein and enzyme technologies could lead to innovative treatments for patients in the future.
Organizations
Indications
Drugs
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.



