Exero Medical's Pivotal Clinical Study of xBar Approved by WCG in the US
17 Jan 2024
Drug ApprovalBreakthrough Therapy
Enrollment to commence in Q1 2024
OR YEHUDA, Israel, Jan. 17, 2024 /PRNewswire/ -- Exero Medical's prospective, blinded, multi-center study of xBar has been approved by the WCG.
The study, expected to commence during Q1 2024 at several leading US and Israeli hospitals, will enroll about 190 patients undergoing colorectal resection. It will evaluate the performance of Exero Medical's flagship device, xBar, in accurately monitoring tissue healing during post-operative recovery to enable the early detection of major complications, such as anastomotic leaks, and the application of enhanced recovery protocols.
Continue Reading
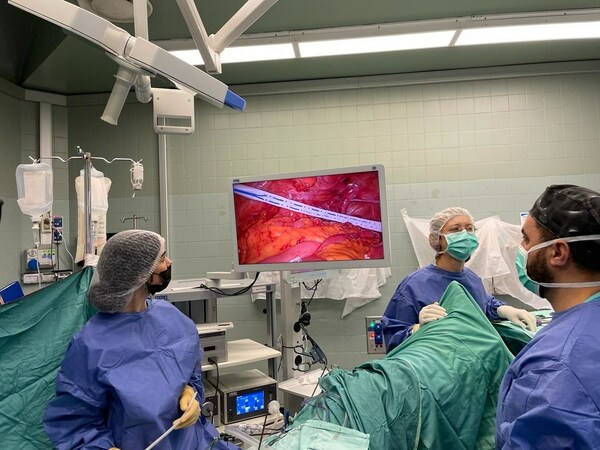
Preview
Source: PRNewswire
xBar in a clinical setting. Photo credit: Exero Medical
This new approach to personalized care for patients undergoing gastrointestinal surgery is intended to enhance surgical outcomes and reduce hospital costs.
This study follows a prior successful clinical study presented in 2023 at the SAGES' Next Big Thing Technology Session.
Prof. Patricia Sylla MD, FACS, FASCRS, System Chief of Colon and Rectal Surgery for the Mount Sinai Health System, said, "Our goal is to detect complications earlier than we do now and minimize unnecessary stays at the hospital. The xBar system can potentially provide us with a new source of data to support implementation of such enhanced recovery protocols." Mount Sinai is among several leading medical centers planning to enroll patients in the study.
Exero Medical CEO Dr. Erez Shor said, "We have developed the xBar system as a way to translate a wealth of physiological data, which has been hidden to date for the most part, into tangible, actionable clinical data. Our goal is to empower the clinical team to provide precise, personalized care on time, avoid conditions that are likely to become dire and optimize hospital stays."
About Exero Medical
Exero Medical was founded in 2018 by MEDX Xelerator, an Israeli Innovation Authority incubator, and Clalit Health Services, the largest HMO in Israel. Exero Medical's goal is to provide high-quality, actionable post-surgery tissue healing monitoring that can save lives, improve prognosis, and reduce costs. The company's flagship product, xBar, addressing a $7B market, received FDA Breakthrough Designation and is in clinical studies towards a commercial launch in 2025.
For more information, please visit https://www.exeromedical.com/
Follow Exero Medical on LinkedIn
Press Contact
Marjie Hadad
General Manager
Must Have Communications
On behalf of Exero Medical
917-790-1178
marjie@mhc-pr.com
Photo - https://mma.prnewswire.com/media/2320396/x_Bar_Exero_Medical.jpg
SOURCE Exero Medical
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Organizations
Indications
Targets
-Drugs
-Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.




