China’s NMPA grants EUA to CSPC Pharmaceutical’s Covid-19 vaccine
23 Mar 2023
VaccinePhase 1mRNAClinical ResultCell Therapy
Preview
Source: Pharmaceutical Technology
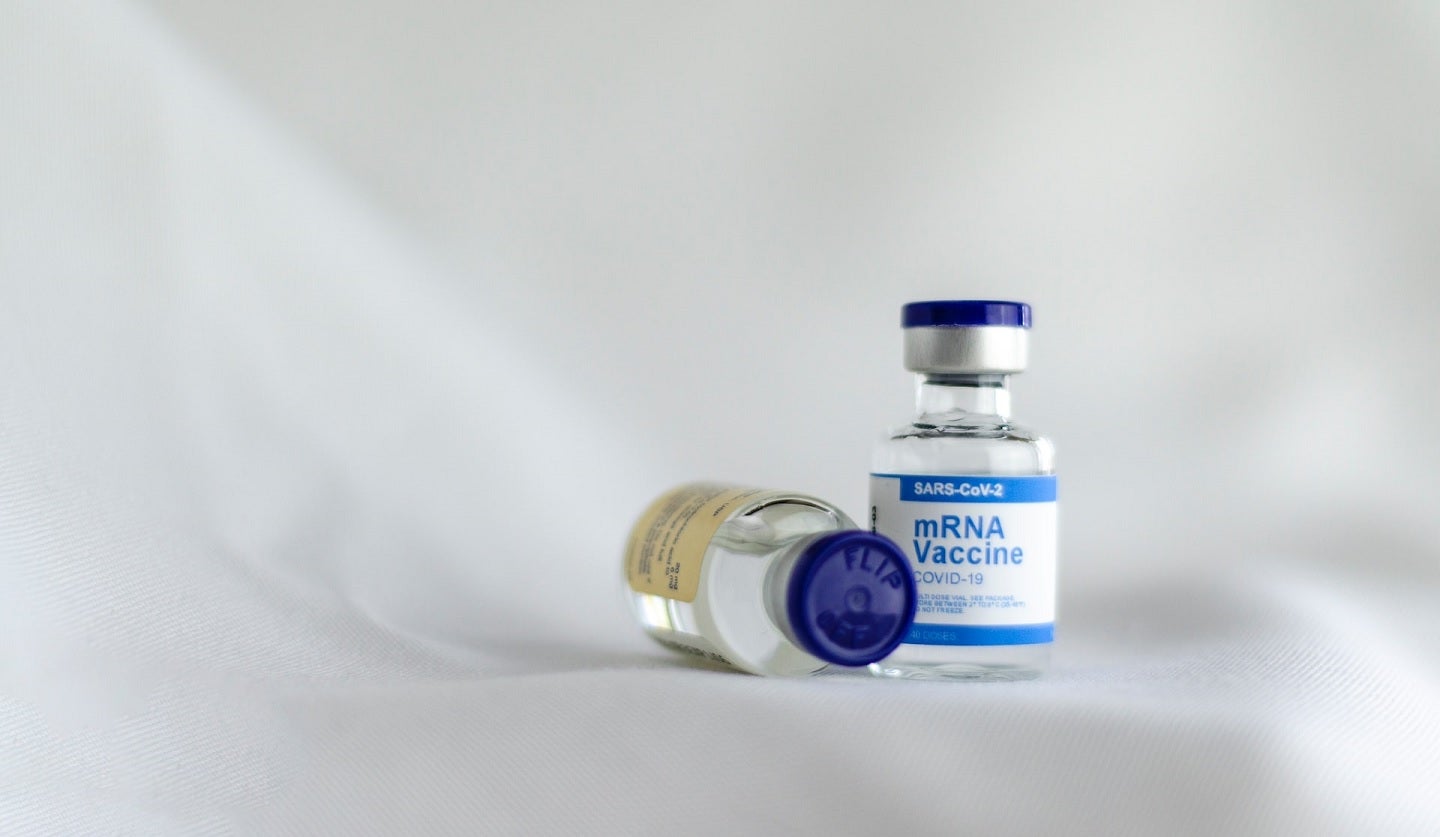
The mRNA Covid-19 vaccineCovid-19 vaccine showed substantially less adverse effects in an elderly group compared with an adult group in clinical trials. Credit: Spencer Davis on Unsplash.

Preview
Source: Pharmaceutical Technology
China’s National Medical Products Administration (NMPA) has granted emergency use authorisation (EUA) for CSPC Pharmaceutical Group’s messenger RNA (mRNA) vaccine, SYS6006, to treat Covid-19.
With this regulatory approval, CSPC Pharmaceutical is claimed to be the first company to receive approval for providing an mRNA vaccine in the country.
The latest authorisation from the Chinese health authority comes as cases of Covid-19 decline after a recent surge.
The independently developed SYS6006 vaccine has been designed to target some major Omicron variants.
In April, CSPC Pharmaceutical secured emergency clinical trial approval to conduct trials of the mRNA vaccine.
With more than 5,500 participants, the company has completed Phases I and II, along with heterologous booster vaccination clinical studies in the country.
In the trials, the vaccine demonstrated substantially fewer adverse effects on a group of elderly subjects compared with an adult group. In a study of 4,000 participants receiving a booster vaccination, it also showed an 85.3% efficacy after 14 to 28 days.
Injection site pain and grade 1 and grade 2 fever were reported to be the most common adverse events.
The mRNA vaccine’s booster dose also demonstrated a good neutralisation effect against Omicron subvariants BA.5, BF.7, BQ.1.1, XBB.1.5 and CH.1.1 in clinical trials.
The company reported a “good” safety profile for the vaccine in August last year.
The BBC reported that laboratories in China have sought to create an mRNA vaccine for years, after the country refused to approve foreign-developed vaccines for widespread domestic use.
The Chinese vaccines were found to be less effective in preventing deaths and severe illness compared to the mRNA vaccines in the studies.
Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.
Editorial content is independently produced and follows the highest standards of journalistic integrity. Topic sponsors are not involved in the creation of editorial content.
Free Whitepaper
Optimise your cell therapy process: a guide to cell thawing
Typically carried out at the point of care, errors in cell therapy thawing could compromise treatment efficacy, leading to significant patient impact as well as high costs and a compromised reputation for the product’s developer.
This guide addresses how cell thawing has historically developed into the new techniques used today, along with the physical and biological implications of key metrics and components such as warming rate and ice structure. Also included are reviews of key studies from scientific literature and a consideration of the interactions between cooling and warming rates, as applicable to cell and gene therapies.

Preview
Source: Pharmaceutical Technology
-->
By clicking the Download Free Whitepaper button, you accept the terms and conditions and acknowledge that your data will be used as described in the Cytiva Thematic privacy policy
By downloading this Whitepaper, you acknowledge that we may share your information with our white paper partners/sponsors who may contact you directly with information on their products and services.
Visit our privacy policy for more information about our services, how we may use, process and share your personal data, including information on your rights in respect of your personal data and how you can unsubscribe from future marketing communications. Our services are intended for corporate subscribers and you warrant that the email address submitted is your corporate email address.
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Organizations
Targets
-Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.



