J&J's Ethicon Faces Mega Electrosurgery Electrode Recall
11 Jul 2023
Clinical Result
Several types of Megadyne electrodes used in electrosurgery and manufactured by Johnson & Johnson's Ethicon business are being recalled due to the risk of serious burn injuries to patients.
According to the FDA recall notice, the company reported that 63 patients (pediatric and adult) received burn injuries from the Mega 2000 and Mega Soft electrodes. The burns may be as serious as third-degree burns requiring medical intervention and may lead to a longer hospital stay, scarring, and potentially more surgeries for patients, FDA noted.
The Class I recall includes 21,200 devices distributed in the United States between March 11, 2021, and May 9, 2023. The company initiated the recall June 1.
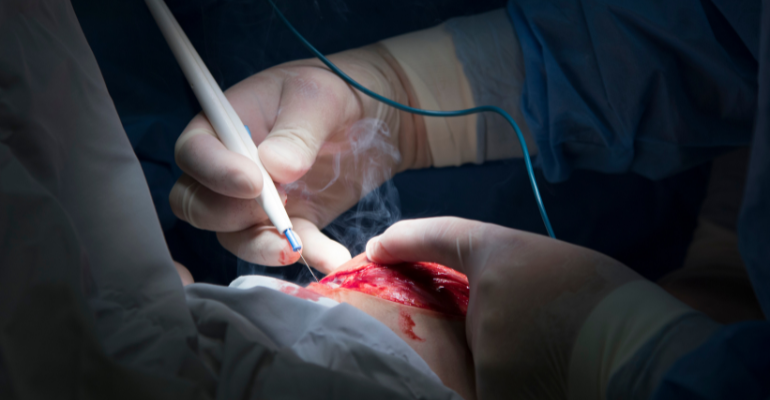
Mega 2000 and Mega Soft reusable patient return electrodes are soft pads used during electrosurgery. During electrosurgery, an electric current is used to heat or cut tissue or to stop bleeding. The electric current is generated by an electrosurgical generator and delivered to the tissue with a small pen-like attachment (like the one shown in the image below). A return electrode pad contacts the skin of the laying patient during use and conducts the electric current from the patient’s tissue back to the electrosurgical unit, or generator, to reduce the risk of excessive heating.
Image credit: vzmaze / iStock via Getty Images
Preview
Source: mddionline
Megadyne sent an urgent medical device correction letter to customers that included the following recommended actions:
Share the notification with all users involved in Mega 2000 and Mega Soft electrodes cleaning, operating room and patient setup, and device operation during procedures.
Confirm that personnel using the electrodes are following the instructions for use.
Post visual aids for cleaning and care and setup, both of which are attached to the letter, near the operating room to remind staff about instructions for cleaning and setup.
Contact any facilities where products may have been shared.
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Targets
-Drugs
-Chat with Hiro
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.





