Multi Radiance Medical Breaks New Ground in Fibromyalgia Pain Relief with the First FDA-Cleared Therapeutic Laser
01 Feb 2024
Clinical Result
Preview
Source: Business Wire
'FibroLux, as one of the first medical devices cleared for fibromyalgia, represents a transformative paradigm shift, providing life-changing relief without side effects'

Preview
Source: Business Wire
SOLON, Ohio--(BUSINESS WIRE)--Pioneering the frontier of super-pulsed laser technology, Multi Radiance Medical highlights the impact of FibroLux Therapeutic Laser, one of the first and only FDA-cleared therapeutic lasers designed to alleviate pain specifically for individuals battling Fibromyalgia Syndrome. Cleared by the FDA in September 2022 and officially launched late 2023, FibroLux represents a significant milestone in addressing the challenges of Fibromyalgia.
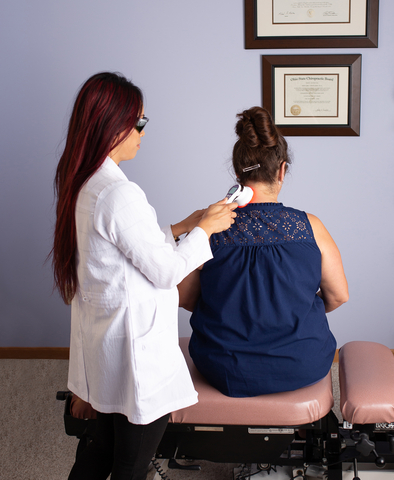
Fibromyalgia affects an estimated 5 million to 8 million Americans and is known for causing chronic widespread pain and tenderness, with limited treatment options, often with adverse side effects. FibroLux Therapy Laser emerges as a cutting-edge, non-invasive, and drug-free solution offering rapid and effective relief by using light energy, in the form of photons to penetrate cells, increasing blood circulation and shutting down pain signals. Clinically proven with minimal side effects, FibroLux is a portable, handheld device that precisely targets 18 tender points, delivering immediate relief in just 2 minutes per point, and an average treatment duration of 6-10 minutes.
“FibroLux, as one of the first medical devices cleared for fibromyalgia, represents a transformative paradigm shift, providing life-changing relief without side effects,” said Max Kanarsky, President and CEO of Multi Radiance Medical. “This laser technology empowers individuals to perform daily activities free from the burden of pain, offering a beacon of hope for those handicapped by fibromyalgia. Recent positive reception at medical conferences underscores the importance of raising awareness among both patients and clinicians.”
FibroLux has exhibited remarkable outcomes in clinical trials, boasting a 52% reduction in tender points, a 53% decrease in pain, and a 45% enhancement in Fibromyalgia Impact Questionnaire scores. In the ground-breaking peer-reviewed study, “Photobiomodulation therapy combined with static magnetic field is better than placebo in patients with fibromyalgia: a randomized placebo-controlled trial,” 86.67% of subjects treated with FibroLux met success criteria, significantly surpassing the 48.89% success rate in the placebo group.
FibroLux stands at the forefront of innovation in Fibromyalgia treatment, enhancing the quality of life for individuals living with chronic pain.
Multi Radiance Medical has remained dedicated to advancing cutting-edge medical solutions for over 15 years, and FibroLux exemplifies their commitment to providing effective, non-invasive, and clinically proven therapies that empower individuals to reclaim an active and pain-free lifestyle. MRM's technology has been the subject of 59+ peer-reviewed articles since inception, and the company has actively engaged in over 35 global clinical trials.
For more information about Multi Radiance Medical and their wide array of super-pulsed laser technologies, visit www.multiradiance.com.
About Multi Radiance Medical
Multi Radiance Medical is a leading medical device company that develops and manufactures FDA-cleared laser therapy equipment for the temporary relief of acute/chronic pain, muscle stiffness, arthritis, muscle spasms, chronic neck and shoulder pain of musculoskeletal origin, and pain associated with fibromyalgia. Multi Radiance Medical has served rehabilitation, chiropractic, physiatry, sports medicine and veterinary markets for 20 years and the technology is utilized in more than 30 countries.
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Organizations
Indications
Targets
-Drugs
-Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.



