CIRM grants funds to Ossium Health for OSSM-007 development
01 May 2023
Cell TherapyClinical StudyImmunotherapyGene Therapy
Preview
Source: Pharmaceutical Technology
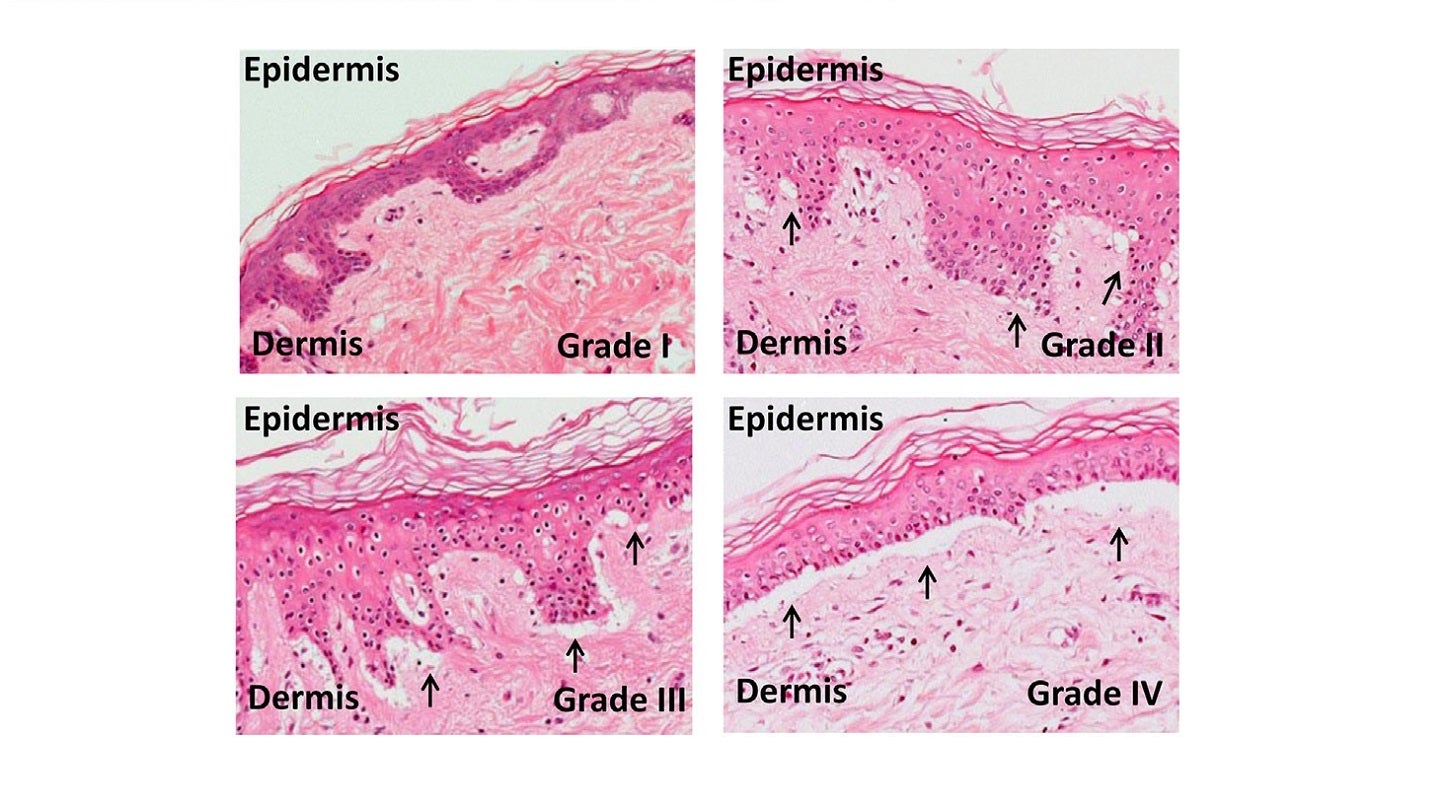
Micrographs of grades of skin graft-versus-host-disease. Credit: Sakhila Ghimire, Daniela Weber, Emily Mavin,Xiao nong Wang,Anne Mary Dickinson and Ernst Holler1 / commons.wikimedia.org.

Preview
Source: Pharmaceutical Technology
The California Institute of Regenerative Medicine (CIRM) has awarded $3.46m Clinical Stage Research Programme (CLIN1) grant to Ossium Health for advancing OSSM-007 development to treat steroid-refractory acute graft versus host disease (GVHD).
OSSM-007 is the interferon-gamma primed mesenchymal stem cell product of Ossium Health.
The grant will help in expediting pre-clinical and manufacturing activities of the product.
The company intends to commence clinical study activities by the end of this year.
Acute GVHD occurs following allogeneic hematopoietic stem cell transplants (HCT).
It is a reaction where immunocompetent donor cells (the graft) identify and attack the tissues of a recipient.
According to Ossium Health, nearly 30 to 60% allogeneic HCT recipients develop acute GVHD and approximately half of them will become refractory to systemic steroid therapy.
Ossium Health CEO and co-founder Kevin Caldwell said: “We’re thrilled to partner with CIRM and appreciate their ongoing commitment to advancing research in stem cell-based therapeutics.
The company uses its deceased donor bone marrow banking platform for developing cell therapies.
These therapies are being assessed to treat orthopaedic trauma, haematologic diseases, and organ rejection.
In March this year, the US Food and Drug Administration (FDA) accepted Ossium Health’s Investigational New Drug (IND) application for OSSM-001 to treat refractory perianal fistulas in Crohn’s disease patients.
Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.
Editorial content is independently produced and follows the highest standards of journalistic integrity. Topic sponsors are not involved in the creation of editorial content.
Free Whitepaper
Optimise your cell therapy process: a guide to cell thawing
Typically carried out at the point of care, errors in cell therapy thawing could compromise treatment efficacy, leading to significant patient impact as well as high costs and a compromised reputation for the product’s developer.
This guide addresses how cell thawing has historically developed into the new techniques used today, along with the physical and biological implications of key metrics and components such as warming rate and ice structure. Also included are reviews of key studies from scientific literature and a consideration of the interactions between cooling and warming rates, as applicable to cell and gene therapies.
By Cytiva Thematic

Preview
Source: Pharmaceutical Technology
-->
By downloading this case study, you acknowledge that GlobalData may share your information with Cytiva Thematic and that your personal data will be used as described in their Privacy Policy
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Organizations
Indications
Targets
Drugs
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.





