SK bioscience’s typhoid vaccine receives WHO prequalification
26 Feb 2024
VaccinePhase 3Clinical Result
Preview
Source: Pharmaceutical Technology
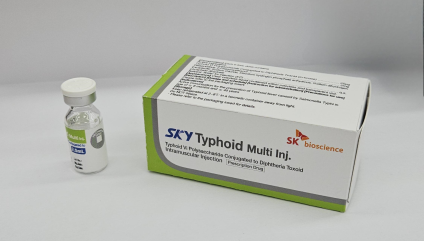
SK bioscience developed the SKYTyphoid vaccine along with the International Vaccine Institute (IVI). Credit: SK bioscience.
SK bioscience has achieved a significant milestone with its typhoid conjugate vaccine, SKYTyphoid, receiving prequalification (PQ) certification from the World Health Organization (WHO).
The WHO PQ system is a rigorous process that ensures the safety and efficacy of vaccines by assessing their manufacturing process, quality and clinical trial data.
The development of SKYTyphoid was a collaborative effort with the International Vaccine Institute (IVI) and received support from the Bill & Melinda Gates Foundation.
SKYTyphoid employs the purified Vi polysaccharide-diphtheria toxoid conjugate method, which is safe for infants and children aged six months to two years.
See Also:Neurocrine Biosciences sees highest patent filings and grants during December in Q4 2023

Preview
Source: Pharmaceutical Technology
Caribou Biosciences sees highest patent filings and grants during November in Q4 2023

Preview
Source: Pharmaceutical Technology
It provides comparable immunogenicity and long-term protection with a single dose, as against current oral live or polysaccharide typhoid vaccines.
In 2022, SKYTyphoid obtained an export licence from the Korean Ministry of Food and Drug Safety, following a global clinical trial.
The Phase III study involved 2,160 subjects aged six months to 45 years in Nepal, showcasing a favourable immunogenicity and safety profile.
The results indicated that SKYTyphoid’s performance was on par with another WHO PQ-certified polysaccharide-protein conjugate typhoid vaccine.
SK bioscience CEO Jaeyong Ahn stated: “We are pleased that our global collaboration to address the global vaccine supply imbalance and improve public health has been recognised by the WHO PQ certification.
“We will make every effort to rapidly supply SKYTyphoid by obtaining approvals in countries.”
With the WHO PQ designation, SK bioscience is set to target global markets, focusing on public procurement in typhoid-endemic countries with high demand.
IVI director general Dr Jerome Kim stated: “Typhoid fever is more prevalent in warmer temperatures, and climate change and the worrying rise of antimicrobial resistance are only adding to the threat of the disease. Vaccination is critical to effective prevention and control of the disease. In collaboration with SK bioscience and other partners, IVI will continue endeavours to make this vaccine accessible to people who need it the most.”
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Indications
Targets
-Drugs
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.





