Pramand Launches CraniSeal™ Dural Sealant to Give Surgeons a New Option for Dural Repair
28 Nov 2023
Drug Approval
BEDFORD, Mass., Nov. 28, 2023 /PRNewswire/ -- Pramand LLC announced today the US commercial launch of the CraniSeal™ Dural Sealant System. CraniSeal is indicated for use in patients ≥ 18 years of age as an adjunct to sutured dural repair during cranial surgery to provide watertight closure.1
Continue Reading
Preview
Source: PRNewswire
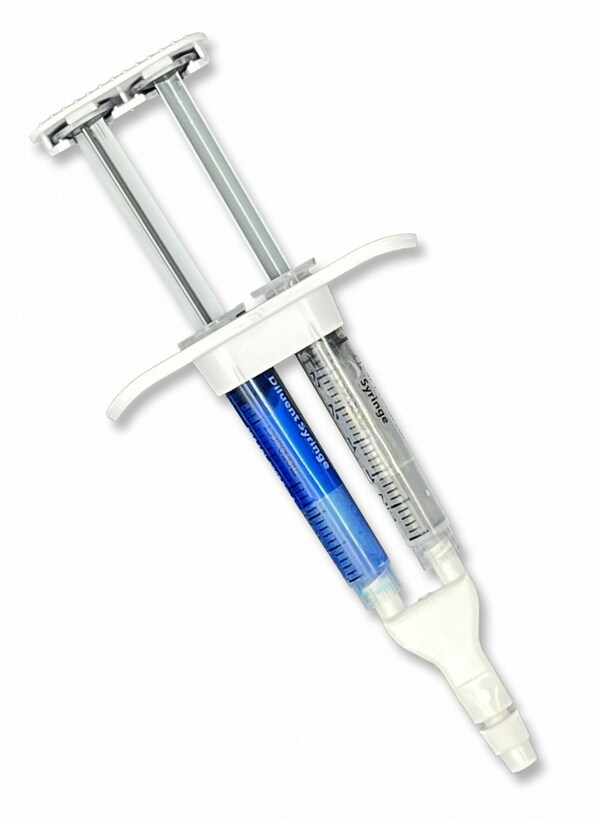
Assembled CraniSeal Dural Sealant
CraniSeal Dural Sealant is an absorbable polyethylene glycol (PEG) hydrogel that is applied with an applicator over sutures. This sealant prevents cerebrospinal fluid from leaking out of a cranial surgery incision site. The underlying technology has been used for two decades in a variety of medical applications.1
Each year, approximately 160,000 cranial procedures are conducted in the US for reasons such as tumor resection, traumatic injury, and cerebrovascular disease. The volume of these procedures is growing by 3 – 5% annually. Cerebrospinal fluid leaks are one common complication of cranial surgery and can result in a longer hospital stay (+6 days) and higher medical costs (+$50,000).2
Amar Sawhney, PhD, Pramand's CEO, remarked, "We are excited to offer neurosurgeons and hospitals a choice for cranial dural sealing with CraniSeal which uses proven, highly effective technology with a long record of safety and efficacy." Dr. Sawhney previously invented DuraSeal® Dural Sealant, which has become the market-leading dural sealant globally.
The Pramand team achieved approval for CraniSeal, a Class 3 device that required Pre-Market Approval (PMA), in just 3.5 years. A typical Class 3 device takes 7 years to achieve approval through the PMA process.3
For more information about CraniSeal, please visit https://www.craniseal.com/.
About Pramand LLC:
Founded in 2020, Pramand develops implantable medical devices from novel, proprietary hydrogel materials that improve patient outcomes while lowering the cost of therapy. Pramand's team has launched over ten medical device companies based on hydrogel technology, and these companies have generated over $4B in value and exits and have helped over 5 million patients worldwide achieve better outcomes. For more information about Pramand, please visit http://pramandllc.com/.
1. PRA-MKT-0004, data on file.
2. Varshneya, K., Rodrigues, A. J., Medress, Z. A., Stienen, M. N., Grant, G. A., Ratliff, J. K., & Veeravagu, A. (2019). Risks, costs, and outcomes of cerebrospinal fluid leaks after pediatric skull fractures: a MarketScan analysis between 2007 and 2015, Neurosurgical Focus FOC, 47(5), E10.
3. A deep dive into the medical device development process. Within3. September 30, 2022. Accessed November 27, 2023. https://within3.com/guides/a-deep-dive-into-the-medical-device-development-process.
PRA-MKT-0005, Rev A
SOURCE Pramand
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Organizations
-Indications
Targets
-Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.


