Gan & Lee Pharmaceuticals to Present Groundbreaking Data on Three Innovative Products at the American Diabetes AssociationDiabetes Association's 84th Scientific Sessions
18 Jun 2024
Drug Approval
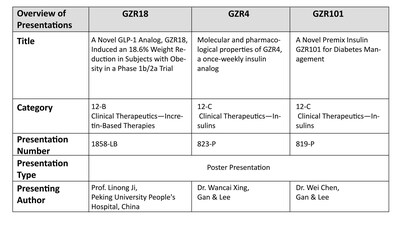
A phase 1b/2a trial evaluated once- and bi-weekly GZR18, a novel GLP-1 analog, in Chinese subjects with obesity/overweight
Pre-clinical studies evaluated the molecular and pharmacological properties of GZR4, an investigational once-weekly insulin analog
BRIDGEWATER, N.J., June 17, 2024 /PRNewswire/ -- Gan & Lee Pharmaceuticals (Shanghai Stock Exchange: 603087) announced today the presentation of three abstracts featuring their investigational products at the American Diabetes AssociationDiabetes Association's (ADA's[1]) 84th Scientific Sessions. The meeting will be held in-person and virtually from June 21-24, 2024, in Orlando, Florida .
All abstracts will be published on the journal Diabetes® website. Data from the selected studies will be presented live on June 22, from 12:30-1:30 PM EDT in the Poster Hall.
Preview
Source: PRNewswire
Preview
Source: PRNewswire

Preview
Source: PRNewswire

Preview
Source: PRNewswire
The above abstracts and presentations represent the data that will be showcased or published by Gan & Lee. This press release includes forward-looking statements regarding investigational products currently in development by Gan & Lee. It is important to note that there are risks associated with drug development, and there is no guarantee that future studies will produce results consistent with those presented at the ADA's 84th Scientific Sessions.
References:
[1]. ADA's 84th Scientific Sessions. https://professional.diabetes.org/scientific-sessions
[2]. Zhang M, Zhang Y, Peng X, et al. GZR18, a novel long-acting GLP-1 analog, demonstrated positive in vitro and in vivo pharmacokinetic and pharmacodynamic characteristics in animal models. Eur J Pharmacol. 2022;928:175107. doi:10.1016/j.ejphar.2022.175107
About Gan & Lee
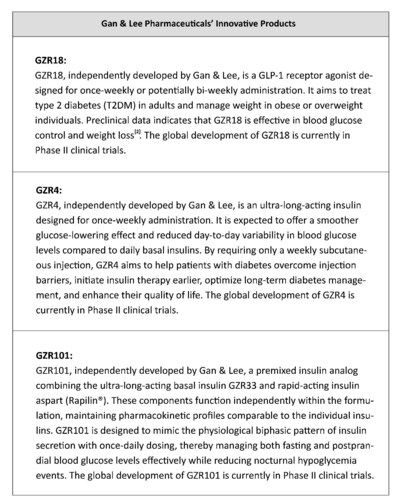
Gan & Lee Pharmaceuticals developed the first Chinese domestic insulin analog. Currently, Gan & Lee has six core insulin products, including five insulin analog varieties: long-acting glargine injection (Basalin®), fast-acting lispro injection (Prandilin™), fast-acting aspart injection (Rapilin®), mixed protamine zinc lispro injection (25R) (Prandilin™25), aspart 30 injection (Rapilin®30), and one human insulin injection - mixed protamine human insulin injection (30R) (Similin®30). The company has two approved medical devices in China, namely reusable insulin injection pen (GanleePen), and disposable pen needle (GanleeFine®).
In China's second Volume Based Procurement (VBP) in 2024, Gan & Lee Pharmaceuticals ranked second overall and first among domestic companies in terms of procurement demand for insulin analogs. The company is also making strides in international markets, with the disposable pen needle (GanleeFine®) approved by the US Food and Drug Administration (FDA) in 2020 and received GMP inspection approval from the European Medicines Agency (EMA) in 2024. These achievements significantly boost Gan & Lee's competitiveness in both international and domestic markets.
In the future, Gan & Lee will strive for comprehensive coverage in diabetes treatment. Moving forward with its mission to become a world-class pharmaceutical company, Gan & Lee will also actively develop new chemical entities and biological drugs, focusing on treatments for metabolic diseases, cardiovascular diseases, and other therapeutic areas.
Further Information
[email protected](Media)
[email protected](Business Development)
SOURCE Gan & Lee Pharmaceuticals
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Organizations
Indications
Targets
-Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.