Insilico teams up with Menarini for breast cancer KAT6 inhibitor
05 Jan 2024
Phase 1License out/in
Preview
Source: Pharmaceutical Technology
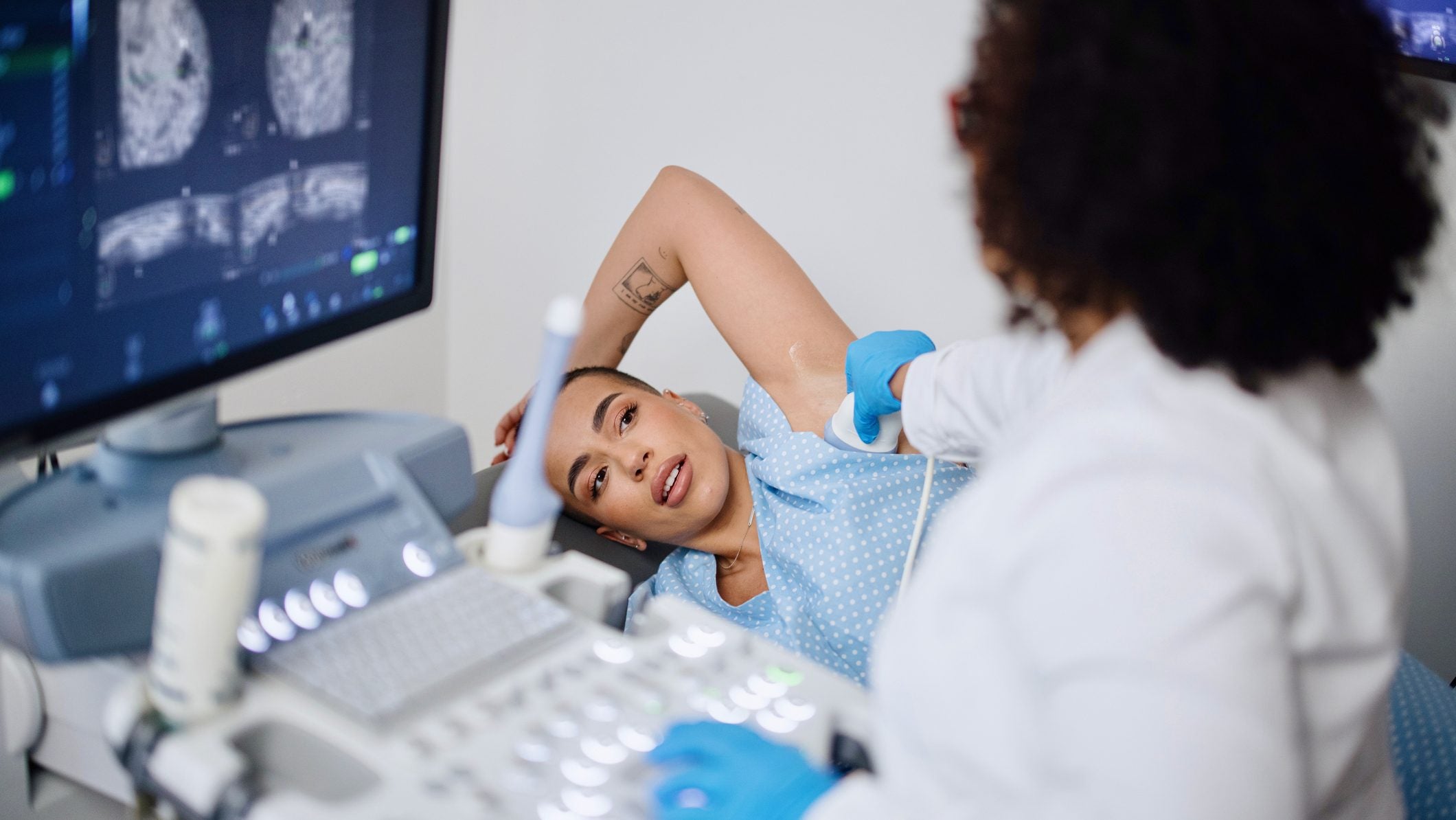
The standard of care for ER+/HER2- breast cancers is endocrine treatments, that may eventually stop working. Credit: Anchiy via Getty Images.
Insilico Medicine has joined forces with MenariniMenarini through its subsidiary Stemline Therapeutics to develop a small molecule KAT6A inhibitor to treat hormone-sensitive cancers, focusing on ER+/HER2- breast cancer.
The molecule has shown positive results in preclinical studies, demonstrating a potent inhibition in multiple CDX and PDX models, as well as a good safety and efficacy profile. Insilico presented data on this development at the San Antonio Breast Cancer Symposium last month.
In September 2019, Insilico announced its AI-powered drug discovery system GENTRL, which was created in alliance with WuXi AppTec, a global open-access R&D and manufacturing platform, as well as professor Alán Aspuru-Guzik, who works in quantum computing and AI in chemistry.
In the announcement accompanying the agreement, Menarini CEO Elcin Barker Ergun said: “Having brought the first innovation in endocrine therapy after almost 20 years to the US and Europe with elacestrant for ER+/HER2- breast cancer patients, our aim is to further augment patient outcomes, and targeting KAT6A can potentially serve that in breast cancer and beyond.”
KAT6A proteins play a crucial role in regulating gene expression and are implicated in various cancers, including poor clinical outcomes of ER+/HER- breast cancers.
Other companies are also looking at the same protein inhibition. Pfizer’s PF-07248144 is under development for the treatment of locally advanced or metastatic ER+/HER2- breast cancer, metastatic castration-resistant prostate cancer and metastatic non-small cell lung cancer (NSCLC).
According to GlobalData’s Pharmaceutical Intelligence Center, PF-07248144 is currently being investigated in a Phase I clinical trial, with a Phase II trial planned.
GlobalData is the parent company of Pharmaceutical Technology.
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Organizations
Targets
Drugs
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.