Olympus Distribution of EndoClot® Showing Steady Adoption in the U.S.
01 Mar 2024
CENTER VALLEY, Pa., March 1, 2024 /PRNewswire/ -- Olympus, a leading global medtech company providing innovative solutions for medical and surgical procedures, announced today that EndoClot products are now used in 49 of the 50 U.S. states as well as the District of Columbia and Guam, since Olympus became the exclusive U.S. distributor of EndoClot hemostatis and submucosal injection solution technologies in 2022.
Continue Reading
Preview
Source: PRNewswire
EndoClot products are used by more than 700 accounts, including most of the country’s top-ranked (by size) Integrated Delivery Networks (IDNs)
More than 5,000 EndoClot kits have been shipped to customers to date. EndoClot products are used by more than 700 accounts, including most of the country's top-ranked (by size) Integrated Delivery Networks (IDNs).
Since their launch, EndoClot products have been discussed in close to 20 relevant professional education courses offered via Olympus Continuum, providing physicians with peer-demonstrations of EndoClot technology capabilities during procedures.
"Olympus knew that EndoClot technology would be important to improving procedure efficiencies and contributing to improved patient outcomes," said Patrick Romano, Vice President, GI Business Unit Leader, Olympus Corporation of the Americas. "But we are nevertheless impressed with the traction that the products have gained in such a short time. The team has been focused on EndoClot since its commercial launch because identifying and treating GI bleeds have been problems in need of optimized solutions for some time."
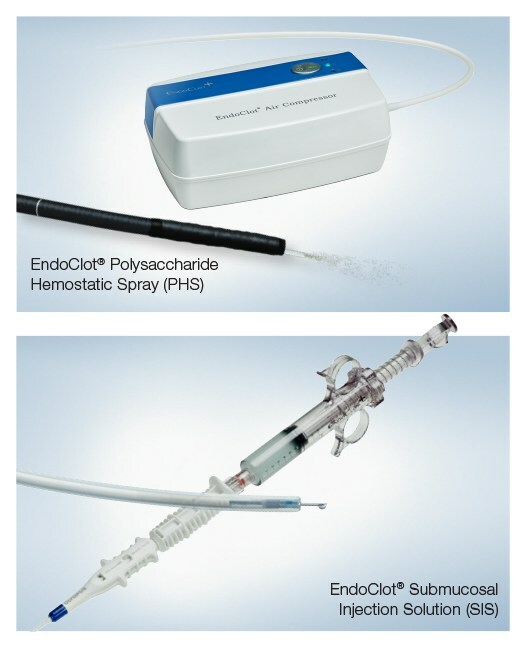
The EndoClot® Polysaccharide Hemostatic Spray (PHS) and EndoClot® Submucosal Injection Solution (SIS) were developed by EndoClot Plus, Inc. (EPI), which joined with Olympus in a U.S. distribution agreement in 2022, extending upon an earlier agreement in the EMEA region. EndoClot PHS and SIS became commercially available in the U.S. in September 2022 and in the Europe, Middle East and Africa (EMEA) region in April 2023. Both products are based on the EndoClot Absorbable Modified Polymer (AMP®) technology, which has demonstrated an excellent safety profile.1 The EndoClot AMP particles work by absorbing water from blood. The dehydration process causes a high concentration of platelets, red blood cells and coagulation proteins, which helps accelerate the body's clotting cascade. AMP particles are biocompatible, bioabsorbable, non-pyrogenic, starch derived and contain no animal or human components.1
"I have been an EndoClot technology user, both PHS and SIS, from almost as soon as it was available, and I am pleased with the results," said Ahmed Saeed, MD, Premier Gastroenterology of Kansas City, Overland Park, Kansas.* "Patients deserve the technology that can prevent complications from becoming more emergent and requiring follow-up care."
The EndoClot PHS System enables physicians to apply an advanced powder hemostat during a procedure using controlled, consistent air pressure through a portable air compressor. Used for hemostasis of nonvariceal gastrointestinal bleeding, excluding Forrest Ia classification of bleeding, the EndoClot PHS System1:
Is indicated for use within the gastrointestinal (GI) tract and in combination with other conventional techniques, like clipping, for large and diffuse bleeds, such as those occurring in peptic ulcers, post-biopsy, polypectomy, tumor bleeding, as well as post-EMR and ESD;
Allows for easy irrigation with water during procedures;
Provides control of delivery and anti-reflux capability through the applicator design, which can prevent occlusion and treat hard-to-reach bleeds; and
Features an air compressor of small, portable design and provides consistent air pressure to propel powder to the bleeding site, while helping prevent the white-out effect common with CO2 propellant.
Performing hemostasis within the GI tract is a technically demanding procedure and the use of EndoClot PHS and associated devices may result in patient injury including but not limited to inflammatory reaction, bowel rupture and air embolism. EndoClot PHS is not indicated for use in surgical or trauma applications.
The EndoClot SIS System is intended for use in gastrointestinal endoscopic procedures for submucosal lift of polyps, adenomas, early stage cancers or other gastrointestinal mucosal lesions, prior to excision with a snare or endoscopic device. The EndoClot SIS solution1:
Provides a long-lasting, high lift that may create significant mucosal separation allowing for easy dissection;
Accurately delivers to the targeted area owing to use of a specialized spiral syringe design; and
Minimizes residual artifacts that may cause abnormalities during pathological investigations.
Use of a lifting agent during EMR/ESD/POEM and difficult polypectomy and the associated devices may result in patient injury, bleeding and/or perforation.
For more information visit the EndoClot PHS and EndoClot SIS product pages. A new video highlighting use of the EndoClot PHS and EndoClot SIS has been developed to provide more information: https://www.youtube.com/watch?v=QlCaoiM2Eo0.
1. Data on file with Olympus as of 16/Aug/2022
*Dr. Saeed is a paid consultant to Olympus.
About Olympus
At Olympus, we are committed to our purpose of making people's lives healthier, safer and more fulfilling. As a global medical technology company, we partner with healthcare professionals striving to provide best-in-class solutions and services for early detection, diagnosis and minimally invasive treatment, aiming to improve patient outcomes by elevating the standard of care in targeted disease states.
For more than 100 years, Olympus has pursued a goal of contributing to society by producing products designed with the purpose of delivering optimal outcomes for its customers around the world. For more information, visit medical.olympusamerica.com.
SOURCE Olympus Corporation of the Americas
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Organizations
Indications
Targets
-Drugs
-Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.