VB10.16 and Atezolizumab Combination Shows Promise for Advanced Cervical Cancer Treatment
20 Apr 2023
Clinical StudyVaccine
PatSnap Synapse, a novel, AI Powered Drug Competitive Intelligence database. Access to drugs, clinical trials, patents, literature and news in one search
Nykode Therapeutics, a biopharmaceutical company based in Oslo, Norway, has announced positive final results from its Phase 2 clinical trial evaluating the safety and efficacy of VB10.16 in combination with the PD-L1 inhibitor, atezolizumab, for the treatment of advanced or recurrent, non-resectable HPV16-positive cervical cancer.
The trial involved 52 patients with advanced cervical cancer who had previously received chemotherapy. The study aimed to evaluate the safety and efficacy of VB10.16, a therapeutic vaccine that works by stimulating the immune system to target cancer cells, in combination with atezolizumab, a checkpoint inhibitor that blocks PD-L1 to enhance immune responses against cancer.
The results showed that the combination of VB10.16 and atezolizumab was well-tolerated and demonstrated promising clinical activity in patients with advanced cervical cancer. The overall response rate was 40% and DCR 80% with a median progression free survival of 16.9 months and median overall survival more than 25 months (not reached).
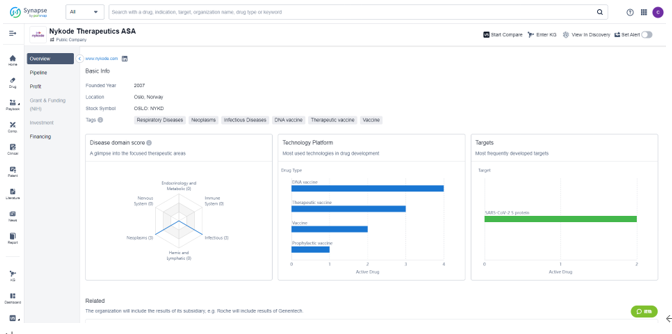
Nykode Therapeutics was founded in 2007 and is focused on developing treatments for respiratory diseases, neoplasms, and infectious diseases. The company's drug development technologies include DNA vaccines, therapeutic vaccines, prophylactic vaccines, and more. Nykode Therapeutics has also been actively developing treatments for SARS-CoV-2 S protein, a frequent target for its drug development efforts.
Preview
Source: SYNAPSE
VB10.16 is a DNA therapeutic vaccine for neoplasms and urogenital diseases, including uterine cervical cancer. It is a product of Nykode Therapeutics and modulates antigen-presenting cells to elicit an immune response against cancer cells. The highest phase of its clinical
trials is Phase 2.
Organizations
Indications
Targets
Drugs
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.



