AstraZeneca signs licence deal with KYM Biosciences for ADC CMG901
24 Feb 2023
License out/inPhase 1ADC
Preview
Source: Pharmaceutical Technology
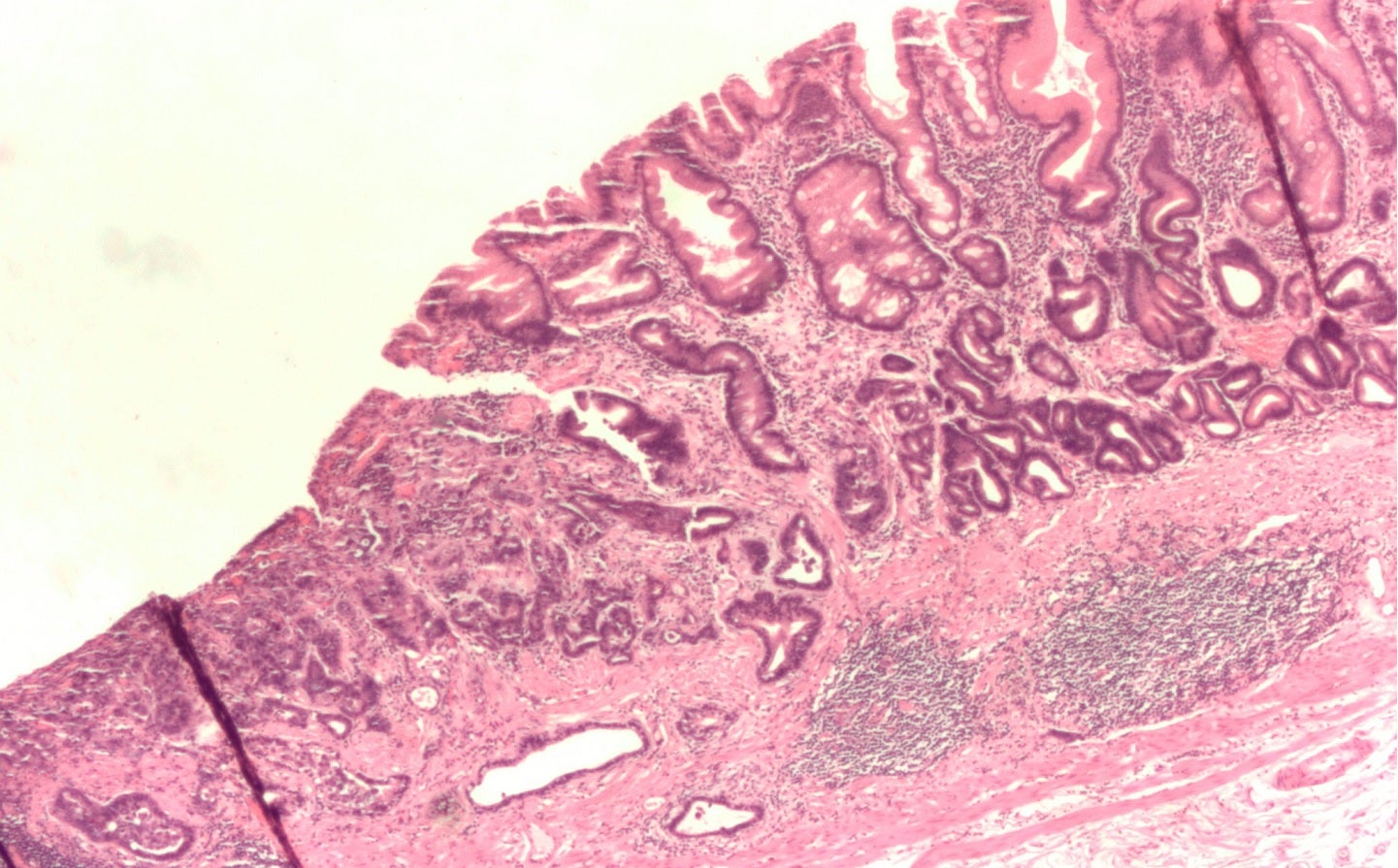
KYM Biosciences’ CMG901 is being developed to treat gastric cancers. Credit: Nephron / Wikimedia.

Preview
Source: Pharmaceutical Technology
AstraZeneca has signed a global exclusive licence agreement with KYM Biosciences for the latter’s Claudin-18.2 antibody-drug conjugate (ADC), CMG901.
Free Whitepaper
PepSetsTM and their Applications
Peptide libraries are a powerful tool in biological research for screening large numbers of peptides in the search for the few, critical bioactive peptides.
Mimotopes PepSets are custom-synthesized peptide libraries supplied unpuried for fast, efcient screening work. These sets of chemically synthesized individual peptides are made
on a small scale using Mimotopes’ unique proprietary parallel array synthesis platform. PepSets comprising hundreds, or even thousands of peptides, are rapidly synthesized and shipped within a few weeks of ordering.
By Mimotopes Thematic

Preview
Source: Pharmaceutical Technology
Submit
Country *
United Kingdom
United States
Afghanistan
Åland Islands
Albania
Algeria
American Samoa
Andorra
Angola
Anguilla
Antarctica
Antigua and Barbuda
Argentina
Armenia
Aruba
Australia
Austria
Azerbaijan
Bahamas
Bahrain
Bangladesh
Barbados
Belarus
Belgium
Belize
Benin
Bermuda
Bhutan
Bolivia
Bosnia and Herzegovina
Botswana
Bouvet Island
Brazil
British Indian Ocean Territory
Brunei Darussalam
Bulgaria
Burkina Faso
Burundi
Cambodia
Cameroon
Canada
Cape Verde
Cayman Islands
Central African Republic
Chad
Chile
China
Christmas Island
Cocos (Keeling) Islands
Colombia
Comoros
Congo
Congo, The Democratic Republic of
The
Cook Islands
Costa Rica
Cote D"ivoire
Croatia
Cuba
Cyprus
Czech Republic
Denmark
Djibouti
Dominica
Dominican Republic
Ecuador
Egypt
El Salvador
Equatorial Guinea
Eritrea
Estonia
Ethiopia
Falkland Islands (Malvinas)
Faroe Islands
Fiji
Finland
France
French Guiana
French Polynesia
French Southern Territories
Gabon
Gambia
Georgia
Germany
Ghana
Gibraltar
Greece
Greenland
Grenada
Guadeloupe
Guam
Guatemala
Guernsey
Guinea
Guinea-bissau
Guyana
Haiti
Heard Island and Mcdonald Islands
Holy See (Vatican City State)
Honduras
Hong Kong
Hungary
Iceland
India
Indonesia
Iran, Islamic Republic of
Iraq
Ireland
Isle of Man
Israel
Italy
Jamaica
Japan
Jersey
Jordan
Kazakhstan
Kenya
Kiribati
Korea, Democratic People"s
Republic of
Korea, Republic of
Kuwait
Kyrgyzstan
Lao People"s Democratic Republic
Latvia
Lebanon
Lesotho
Liberia
Libyan Arab Jamahiriya
Liechtenstein
Lithuania
Luxembourg
Macao
Macedonia, The Former
Yugoslav Republic of
Madagascar
Malawi
Malaysia
Maldives
Mali
Malta
Marshall Islands
Martinique
Mauritania
Mauritius
Mayotte
Mexico
Micronesia, Federated States of
Moldova, Republic of
Monaco
Mongolia
Montenegro
Montserrat
Morocco
Mozambique
Myanmar
Namibia
Nauru
Nepal
Netherlands
Netherlands Antilles
New Caledonia
New Zealand
Nicaragua
Niger
Nigeria
Niue
Norfolk Island
Northern Mariana Islands
Norway
Oman
Pakistan
Palau
Palestinian Territory, Occupied
Panama
Papua New Guinea
Paraguay
Peru
Philippines
Pitcairn
Poland
Portugal
Puerto Rico
Qatar
Reunion
Romania
Russian Federation
Rwanda
Saint Helena
Saint Kitts and Nevis
Saint Lucia
Saint Pierre and Miquelon
Saint Vincent and The Grenadines
Samoa
San Marino
Sao Tome and Principe
Saudi Arabia
Senegal
Serbia
Seychelles
Sierra Leone
Singapore
Slovakia
Slovenia
Solomon Islands
Somalia
South Africa
South Georgia and The South
Sandwich Islands
Spain
Sri Lanka
Sudan
Suriname
Svalbard and Jan Mayen
Swaziland
Sweden
Switzerland
Syrian Arab Republic
Taiwan, Province of China
Tajikistan
Tanzania, United Republic of
Thailand
Timor-leste
Togo
Tokelau
Tonga
Trinidad and Tobago
Tunisia
Turkey
Turkmenistan
Turks and Caicos Islands
Tuvalu
Uganda
Ukraine
United Arab Emirates
United States Minor Outlying
Islands
Uruguay
Uzbekistan
Vanuatu
Venezuela
Viet Nam
Virgin Islands, British
Virgin Islands, U.S.
Wallis and Futuna
Western Sahara
Yemen
Zambia
Zimbabwe
Submit
By clicking the Download Free Whitepaper button, you accept the terms and conditions and acknowledge that your data will be used as described in the Mimotopes Thematic privacy policy
By downloading this Whitepaper, you acknowledge that we may share your information with our white paper partners/sponsors who may contact you directly with information on their products and services.
Visit our privacy policy for more information about our services, how we may use, process and share your personal data, including information on your rights in respect of your personal data and how you can unsubscribe from future marketing communications. Our services are intended for corporate subscribers and you warrant that the email address submitted is your corporate email address.
Thank you.
Please check your email to download the Whitepaper.
KYM Biosciences is a joint venture (JV) established by Chinese biotechnology firm Keymed Biosciences and Lepu Biopharma.
Under the licence deal, AstraZeneca will handle the research and activities related to the development, manufacturing, and commercialisation of CMG901 globally.
CMG901 comprises an anti-Claudin 18.2 monoclonal antibody, a cytotoxic small molecule monomethyl auristatin E (MMAE) and a protease-degradable linker.
It is being developed to treat solid tumours that express the Claudin 18.2 cell surface protein, including gastric cancers.
Currently, the ADC is being assessed in a Phase I clinical trial to treat Claudin 18.2-positive solid tumours, including gastric cancer.
AstraZeneca Oncology R&D Biologics Engineering & Oncology Targeted Delivery senior vice-president Puja Sapra said: “We are excited by the opportunity to accelerate the development of CMG901, a potential new medicine for patients with Claudin18.2-expressing cancers.
“CMG901 strengthens our growing pipeline of antibody-drug conjugates and supports our ambition to expand treatment options and transform outcomes for patients with gastrointestinal cancers.”
According to the agreement, KYM Biosciences will receive $63m in upfront payment on the closing of the transaction along with up to $1.1bn in further development and sales-related milestones from AstraZeneca.
The company will also receive tiered royalties up to low double digits.
The transaction, subject to regulatory clearances and customary closing conditions, is anticipated to be concluded in the first half of this year.
Early R&D projects coverage on Pharmaceutical Technology is supported by Mimotopes.
Editorial content is independently produced and follows the highest standards of journalistic integrity. Topic sponsors are not involved in the creation of editorial content.
Free Whitepaper
PepSetsTM and their Applications
Peptide libraries are a powerful tool in biological research for screening large numbers of peptides in the search for the few, critical bioactive peptides.
Mimotopes PepSets are custom-synthesized peptide libraries supplied unpuried for fast, efcient screening work. These sets of chemically synthesized individual peptides are made
on a small scale using Mimotopes’ unique proprietary parallel array synthesis platform. PepSets comprising hundreds, or even thousands of peptides, are rapidly synthesized and shipped within a few weeks of ordering.
By Mimotopes Thematic

Preview
Source: Pharmaceutical Technology
Submit
Country *
United Kingdom
United States
Afghanistan
Åland Islands
Albania
Algeria
American Samoa
Andorra
Angola
Anguilla
Antarctica
Antigua and Barbuda
Argentina
Armenia
Aruba
Australia
Austria
Azerbaijan
Bahamas
Bahrain
Bangladesh
Barbados
Belarus
Belgium
Belize
Benin
Bermuda
Bhutan
Bolivia
Bosnia and Herzegovina
Botswana
Bouvet Island
Brazil
British Indian Ocean Territory
Brunei Darussalam
Bulgaria
Burkina Faso
Burundi
Cambodia
Cameroon
Canada
Cape Verde
Cayman Islands
Central African Republic
Chad
Chile
China
Christmas Island
Cocos (Keeling) Islands
Colombia
Comoros
Congo
Congo, The Democratic Republic of
The
Cook Islands
Costa Rica
Cote D"ivoire
Croatia
Cuba
Cyprus
Czech Republic
Denmark
Djibouti
Dominica
Dominican Republic
Ecuador
Egypt
El Salvador
Equatorial Guinea
Eritrea
Estonia
Ethiopia
Falkland Islands (Malvinas)
Faroe Islands
Fiji
Finland
France
French Guiana
French Polynesia
French Southern Territories
Gabon
Gambia
Georgia
Germany
Ghana
Gibraltar
Greece
Greenland
Grenada
Guadeloupe
Guam
Guatemala
Guernsey
Guinea
Guinea-bissau
Guyana
Haiti
Heard Island and Mcdonald Islands
Holy See (Vatican City State)
Honduras
Hong Kong
Hungary
Iceland
India
Indonesia
Iran, Islamic Republic of
Iraq
Ireland
Isle of Man
Israel
Italy
Jamaica
Japan
Jersey
Jordan
Kazakhstan
Kenya
Kiribati
Korea, Democratic People"s
Republic of
Korea, Republic of
Kuwait
Kyrgyzstan
Lao People"s Democratic Republic
Latvia
Lebanon
Lesotho
Liberia
Libyan Arab Jamahiriya
Liechtenstein
Lithuania
Luxembourg
Macao
Macedonia, The Former
Yugoslav Republic of
Madagascar
Malawi
Malaysia
Maldives
Mali
Malta
Marshall Islands
Martinique
Mauritania
Mauritius
Mayotte
Mexico
Micronesia, Federated States of
Moldova, Republic of
Monaco
Mongolia
Montenegro
Montserrat
Morocco
Mozambique
Myanmar
Namibia
Nauru
Nepal
Netherlands
Netherlands Antilles
New Caledonia
New Zealand
Nicaragua
Niger
Nigeria
Niue
Norfolk Island
Northern Mariana Islands
Norway
Oman
Pakistan
Palau
Palestinian Territory, Occupied
Panama
Papua New Guinea
Paraguay
Peru
Philippines
Pitcairn
Poland
Portugal
Puerto Rico
Qatar
Reunion
Romania
Russian Federation
Rwanda
Saint Helena
Saint Kitts and Nevis
Saint Lucia
Saint Pierre and Miquelon
Saint Vincent and The Grenadines
Samoa
San Marino
Sao Tome and Principe
Saudi Arabia
Senegal
Serbia
Seychelles
Sierra Leone
Singapore
Slovakia
Slovenia
Solomon Islands
Somalia
South Africa
South Georgia and The South
Sandwich Islands
Spain
Sri Lanka
Sudan
Suriname
Svalbard and Jan Mayen
Swaziland
Sweden
Switzerland
Syrian Arab Republic
Taiwan, Province of China
Tajikistan
Tanzania, United Republic of
Thailand
Timor-leste
Togo
Tokelau
Tonga
Trinidad and Tobago
Tunisia
Turkey
Turkmenistan
Turks and Caicos Islands
Tuvalu
Uganda
Ukraine
United Arab Emirates
United States Minor Outlying
Islands
Uruguay
Uzbekistan
Vanuatu
Venezuela
Viet Nam
Virgin Islands, British
Virgin Islands, U.S.
Wallis and Futuna
Western Sahara
Yemen
Zambia
Zimbabwe
Submit
By clicking the Download Free Whitepaper button, you accept the terms and conditions and acknowledge that your data will be used as described in the Mimotopes Thematic privacy policy
By downloading this Whitepaper, you acknowledge that we may share your information with our white paper partners/sponsors who may contact you directly with information on their products and services.
Visit our privacy policy for more information about our services, how we may use, process and share your personal data, including information on your rights in respect of your personal data and how you can unsubscribe from future marketing communications. Our services are intended for corporate subscribers and you warrant that the email address submitted is your corporate email address.
Thank you.
Please check your email to download the Whitepaper.
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Organizations
Targets
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.




