/ Not yet recruitingNot ApplicableIIT Effectiveness and Cost-Effectiveness Evaluations of AI-Assisted Diagnostic Software (VeriSee) for Ophthalmic Disease Screening
This study aims to evaluate the effectiveness of an artificial intelligence (AI)-assisted screening system in ophthalmic diagnosis. Using AI-based fundus photography, the system will assist physicians in diagnosing three common eye diseases: age-related macular degeneration, diabetic retinopathy (DR), and glaucoma. The AI system will analyze fundus images from participants and rapidly generate detection results for ophthalmologists' reference in making final diagnoses and clinical decisions. The study will assess the clinical benefits of the AI-assisted diagnostic system, providing scientific evidence to enhance the efficiency of ophthalmic disease diagnosis and treatment.
/ Not yet recruitingNot ApplicableIIT Clinical Effectiveness and Cost-Effectiveness of Real-Time Chest X-Ray Computer-Aided Detection System for Misplaced Endotracheal and Nasogastric Tubes and Pneumothorax in Emergency and Critical Care Settings
Background Advancements in artificial intelligence (AI) have driven significant breakthroughs in computer-aided detection (CAD) for chest X-ray imaging. National Taiwan University Hospital (NTUH) research team previously developed an AI-based emergency Capstone CXR system (MOST 111-2634-F-002-015-, Capstone project), which led to the creation of a chest X-ray module. This chest X-ray module has an established model supported by extensive research and is ready for direct application in clinical trials without requiring additional model training. This study will utilize three submodules of the system: detection of misplaced endotracheal tubes, detection of misplaced nasogastric tubes, and identification of pneumothorax.
Objective This study aims to apply a real-time chest X-ray CAD system in emergency and critical care settings to evaluate its clinical and economic benefits without requiring additional chest X-ray examinations or altering standard care and procedures. The study will evaluate the CAD system's impact on mortality reduction, post-intubation complications, hospital stay duration, workload, and interpretation time, alongside a cost-effectiveness comparison with standard care.
Methods This study adopts a pilot trial and cluster randomized controlled trial design, with random assignment conducted at the ward level. In the intervention group, units are granted access to AI diagnostic results, while the control group continues standard care practices. Consent will be obtained from attending physicians, residents, and advanced practice nurses in each participating ward. Once consent is secured, these healthcare providers in the intervention group will be authorized to use the CAD system. Intervention units will have access to AI-generated interpretations, whereas control units will maintain routine medical procedures without access to the AI diagnostic outputs.
Results The study was funded in September 2024. Data collection is expected to last from January 2025 to December 2027.
Conclusions This study anticipates that the real-time chest X-ray CAD system will automate the identification and detection of misplaced endotracheal and nasogastric tubes on chest X-rays, as well as assist clinicians in diagnosing pneumothorax. By reducing the workload of physicians, the system is expected to shorten the time required to detect tube misplacement and pneumothorax, decrease patient mortality and hospital stays, and ultimately lower healthcare costs.
/ CompletedNot ApplicableIIT A Multi-center, Stepped-wedge, Cluster-randomized Control Trial to Evaluation the Effectiveness of Chlorhexidine to Prevent Catheter-associated Urinary Tract Infection
The study is to investigate whether chlorhexidine (CHG)-based antiseptics is more effective to prevent catheter-related urinary tract infection (CAUTI) among inhospital patients who required Foley catheter insertion. This is a cluster-randomised, step-wedged clinical trial, in which every participated unit will used three different Foley catheter insertion protocols during the study period:
1. Iodine protocol: using 10% povidone-iodine as the primary antiseptic during Foley insertion. This is the routine practice before this study in the participated hospital, as well as many Taiwanese hospitals.
2. CHG protocol: instead of povidone-iodine solution, use 2% aqueous CHG solution as the primary disinfectant during Foley solution.
3. CHG plus protocol: additional to 2% CHG solution, added 0.5% CHG impregnated gel as the lubrication during Foley insertion.
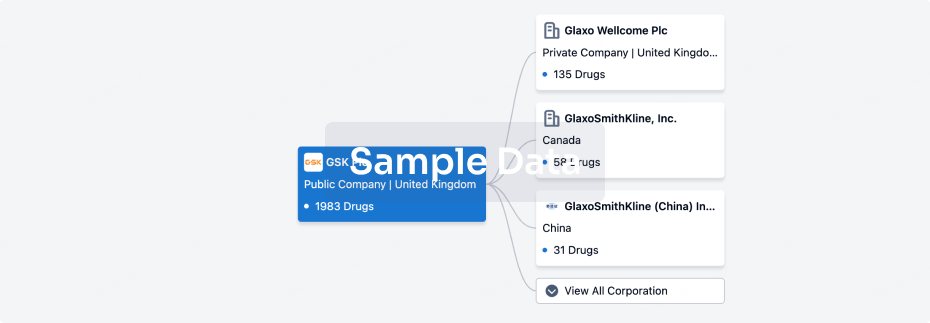
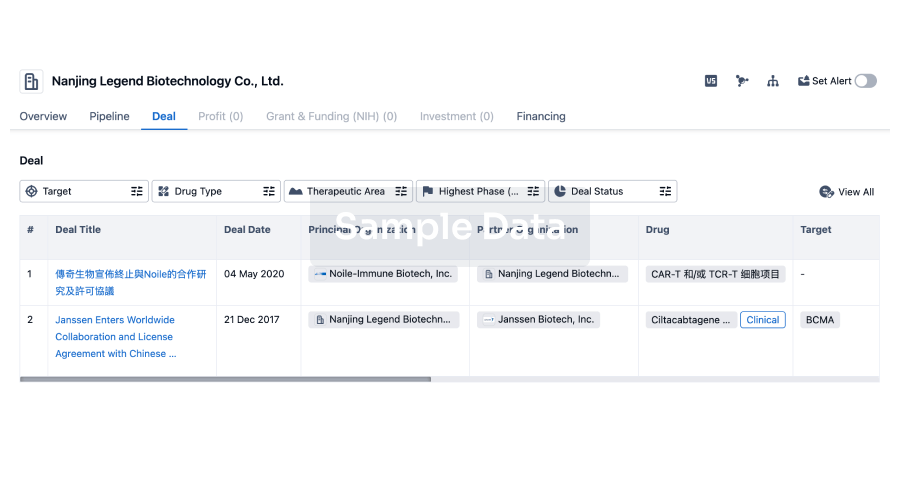
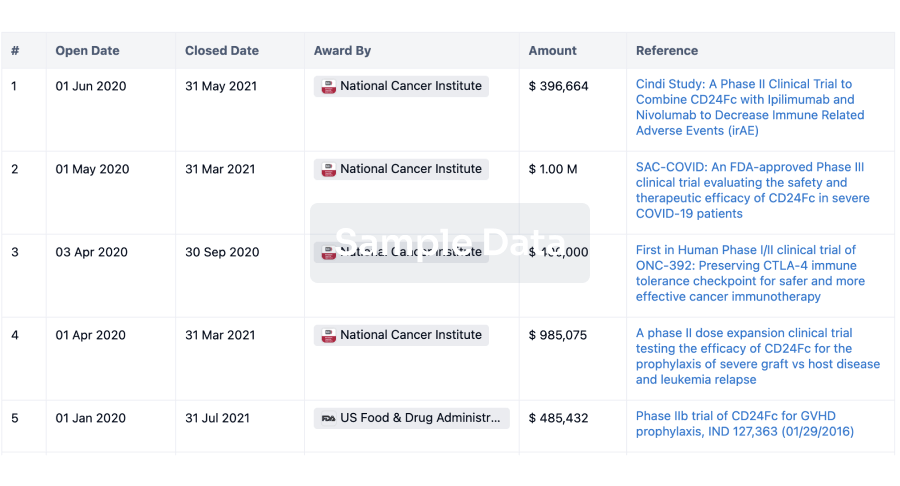
100 Clinical Results associated with Min-Sheng Hospital of Missioncare, Inc.
0 Patents (Medical) associated with Min-Sheng Hospital of Missioncare, Inc.
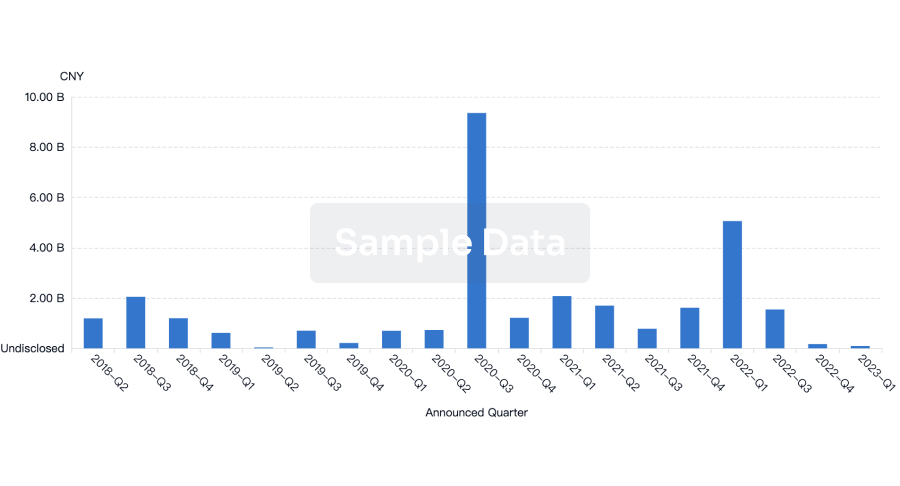
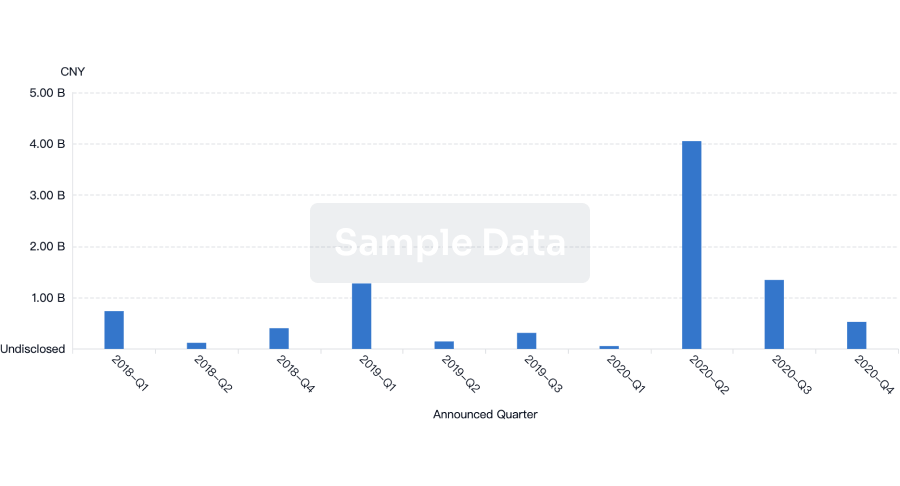
100 Deals associated with Min-Sheng Hospital of Missioncare, Inc.
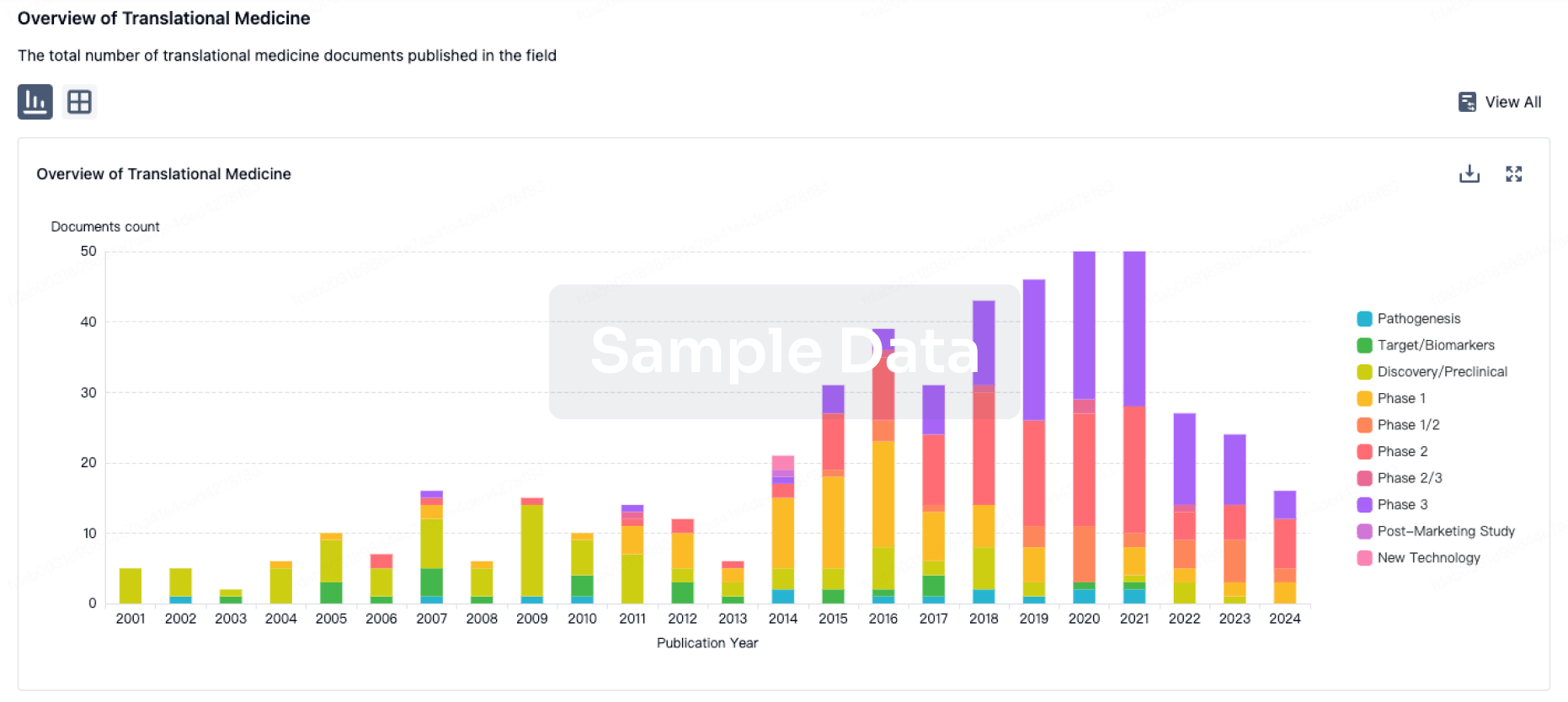
100 Translational Medicine associated with Min-Sheng Hospital of Missioncare, Inc.