Request Demo
Last update 08 May 2025
The Biotechnology Institute
Last update 08 May 2025
Overview
Related
100 Clinical Results associated with The Biotechnology Institute
Login to view more data
0 Patents (Medical) associated with The Biotechnology Institute
Login to view more data
2,097
Literatures (Medical) associated with The Biotechnology Institute01 Aug 2025·Journal of Pharmaceutical and Biomedical Analysis
Too much or too little caffeine? Determination of content in thermogenic capsules by quantitative 1H nuclear magnetic resonance (qNMR)
Article
Author: Schiavenin, Ariane ; Moura, Sidnei ; de Almeida, Rafael Menck ; Longhi, Giovana S ; Armando, Yara Popst ; Crocoli, Luana C
01 Jul 2025·Separation and Purification Technology
Solvent-free extraction of polyhydroxyalkanoates from wet biomass using mechanical cell disruption
Author: Grimm, Thomas ; Neubauer, Peter ; Riedel, Sebastian L. ; Perez, Corinna ; Thiele, Isabel ; Glaeser, Max
01 Jul 2025·Journal of Biotechnology
Fungal elicitors increase cell biomass, pyrroloquinazoline alkaloids production and gene expression levels of biosynthetic pathways in Adhatoda vasica Nees cell cultures
Article
Author: Singh, Bharat ; Sharma, Ram Avtar ; Saxena, Anuja
3
News (Medical) associated with The Biotechnology Institute12 Jun 2023
GAINESVILLE, FL / ACCESSWIRE / June 12, 2023 / Neobiosis, is a leading research and manufacturing biotechnology company focused on pioneering the development of novel therapeutics from perinatal tissues as a new class of medicines. Today, Neobiosis announced that the U.S. Food and Drug Administration (FDA) has approved the Investigational New Drug (IND) application for ViXome™, its drug for the treatment of 'Post COVID Syndrome (AKA "Long Haul COVID Syndrome").
"This is an important milestone for Neobiosis and we are excited to be advancing ViXome, a refined product extracted from thoroughly screened and sterile amniotic fluid obtained at the time of c-section of at-term pregnancies, into clinical development," said Ian A. White, Ph.D., CEO and CSO of Neobiosis. "We are eager to confirm our preclinical data in the clinic and expect to begin enrolling patients in Phase 1 during the second quarter of 2024."
ViXome, an acellular product derived from amniotic fluid, contains a heterogeneous population of growth factors, cytokines, chemokines, microRNAs and exosomes, which in pre-clinical testing has demonstrated potent immunomodulatory and pro-reparative effects.
The Phase I/IIa study will thoroughly investigate the safety and clinical efficacy of ViXome. Neobiosis products are processed in state-of-the-art, FDA-registered and FDA-inspected cGMP-compliant cleanrooms in Gainesville, FL. Neobiosis performs its research and development (R&D) at the University of Florida (UF) Sid Martin Innovate Biotechnology Institute in Alachua, FL., the world-recognized leader in biotechnology incubation.
"We are excited to be one of the first companies in the US to advance a potential therapeutic for the treatment of a devastating disease that already affects over 300 million people worldwide," added Dr. White.
About Neobiosis
Neobiosis is a clinical-stage biopharmaceutical company pioneering the development of perinatal tissue-derived therapeutics, a new class of medicines with the potential to transform the treatment of a wide spectrum of diseases with high unmet medical needs. By leveraging the biology of birth tissues as a natural source of regenerative and immunomodulatory cells, matrix and exosomes, Neobiosis has developed its proprietary platform to augment the innate properties of homeostasis to create a portfolio of novel therapeutic candidates. Neobiosis is dedicated to transparency, safety and efficacy of all of its products developed under FDA oversight.
Contact Information
Ian white
CEO
drwhite@neobiosis.com
3059062345
SOURCE: Neobiosis
View source version on accesswire.com:
22 Jan 2021
Jan. 22, 2021 14:00 UTC
WASHINGTON--(BUSINESS WIRE)-- This week, the Biotechnology Institute announced the launch of a new online afterschool STEM education program “BioDiversity” for 3rd – 8th graders. The fundamental objective of BioDiversity is to engage, excite and educate students of ethnic and cultural backgrounds that are under-represented in the scientific community about their potential to successfully pursue career paths in science. While students will focus on core STEM principles, significant emphasis will be placed on the biological sciences and the potential for biotechnology to help solve some of our most challenging global problems in healthcare, food sustainability and the environment.
The inaugural Winter 12-week program will enroll more than 100 students from East Orange and Camden City, NJ, and the Philadelphia area due to support from Johnson & Johnson. “We are enthusiastic about the launch of the BioDiversity Winter Institute in our surrounding communities,” said Seema Kumar, Global Head, Office of Innovation, Global Public Health and Scientific Engagement at Johnson & Johnson. “Engaging young people from every background in science at the earliest ages is critical to the future of innovation and discovery. Good health and a sustainable future depend on it.”
The Institute has engaged teachers with both educational and industry experience to develop grade appropriate curricula in STEM that includes hands-on experimentation and encourages community engagement through health education and skills development. Classes are delivered five days each week with a mix of programmed and live teacher sessions. Parents, an integral part of the students’ support network during classes, are provided background materials for assistance. Students requiring online access to the program are provided with the necessary technology resources.
“We don’t know why exactly and when any particular student gets excited about science,” said Dr. Larry Mahan, President, Biotechnology Institute. “What we do know is that exposure and experiences need to start at the earliest ages.” With future support from other biotechnology industry leaders, the Institute intends to expand BioDiversity nationwide to include both winter and summer programming in some of the most deserving communities.
###
About the Biotechnology Institute
The Biotechnology Institute is an independent, national nonprofit organization dedicated to education about the present and future impact of biotechnology. Its mission is to engage, excite and educate the public, particularly students and teachers, about biotechnology and its immense potential for solving our most pressing problems in human health, food sustainability and the environment. For more information, visit .
19 Jun 2012
SAN DIEGO, June 19, 2012 /PRNewswire/ -- Althea Technologies, a leading provider of development and manufacturing services for biopharmaceutical products, today announced that Dennis Fenton, Ph.D., will chair Althea's Scientific Advisory Board and be appointed as Board Observer. Dr. Fenton brings 35 years of experience in the biotechnology and pharmaceutical industries to this role. Starting his career at Pfizer Central Research, he subsequently joined Amgen at its inception, where during his 26 year tenure, he helped transform the company from a small biotech to a global leader in the industry. Dr. Fenton's career is distinguished by his strong track record of scientific expertise and innovation; he also holds 5 US patents in microbiology and has published over 50 papers and abstracts.
(Logo: )
"Althea Technologies is very fortunate to be welcoming such an accomplished industry leader to chair its Scientific Advisory Board," said Dr. Magda Marquet, Founder and Co-Chair of Althea Technologies.
"Dennis Fenton has been a pioneer in translating biologic discoveries to high volume commercial production, and his expanded role supports Althea's commitment to provide its clients with state of the art clinical and commercial biologic manufacturing solutions," said Matt Mackowski, Co-Chair of Althea Technologies.
Commenting on his appointment, Dr. Fenton said, "It is an honor to accept the chairman position on Althea's Scientific Advisory Board. I am excited to contribute my experience in microbiology research within the pharmaceutical industry to Althea's continued growth as a provider of quality biologics development and manufacturing services."
Dr. Fenton retired from Amgen in 2008 and has since worked as an independent consultant. Additionally, he has been the member of the boards of Amira, CytomX, Dendreon, Genelux, Genzyme, Kythera, Napo, Xenoport, Rutgers University, the Keck graduate Institute and the Biotechnology Institute. Dr. Fenton received his Ph.D. in Microbiology from Rutgers University.
Althea Technologies' current Scientific Advisory Board consists of Dr. Steve Kay, Dr. Daniel Wang, and Jeff Yuen.
About Althea Technologies
Althea Technologies is a fully integrated, contract development and manufacturing organization located in San Diego, CA providing clinical and commercial product development services. Althea offers cGMP drug product filling in both vials and syringes, and production of microbial-derived recombinant proteins and plasmid DNA. In conjunction with these manufacturing operations, Althea offers comprehensive development services including: upstream and downstream process development, analytical development, lyophilization cycle, complex formulation, product release and ICH-compliant stability testing. Althea's formulation technology platform includes Crystalomics®, a proprietary technology that offers a formulation solution for large molecule products that must be delivered at high concentrations or as sustained release formulations. For more information, visit .
SOURCE Althea Technologies, Inc.
100 Deals associated with The Biotechnology Institute
Login to view more data
100 Translational Medicine associated with The Biotechnology Institute
Login to view more data
Corporation Tree
Boost your research with our corporation tree data.
login
or
Pipeline
Pipeline Snapshot as of 06 Aug 2025
No data posted
Login to keep update
Deal
Boost your decision using our deal data.
login
or
Translational Medicine
Boost your research with our translational medicine data.
login
or
Profit
Explore the financial positions of over 360K organizations with Synapse.
login
or
Grant & Funding(NIH)
Access more than 2 million grant and funding information to elevate your research journey.
login
or
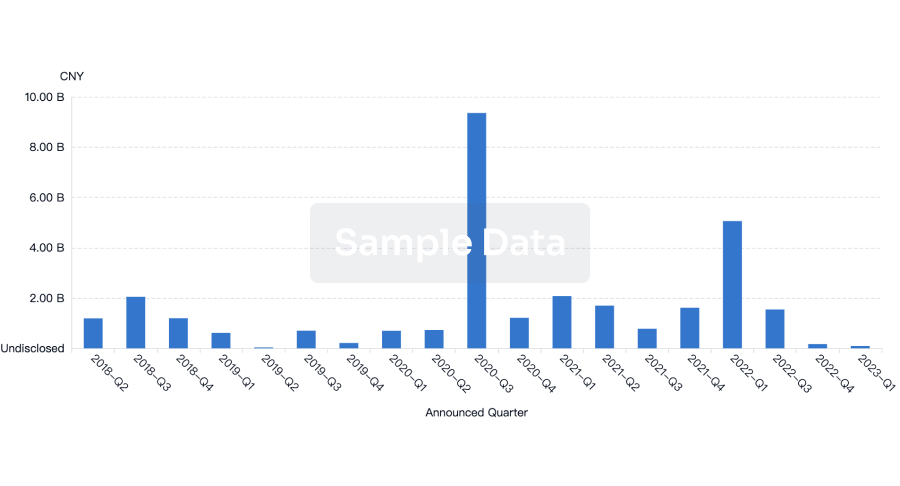
Investment
Gain insights on the latest company investments from start-ups to established corporations.
login
or
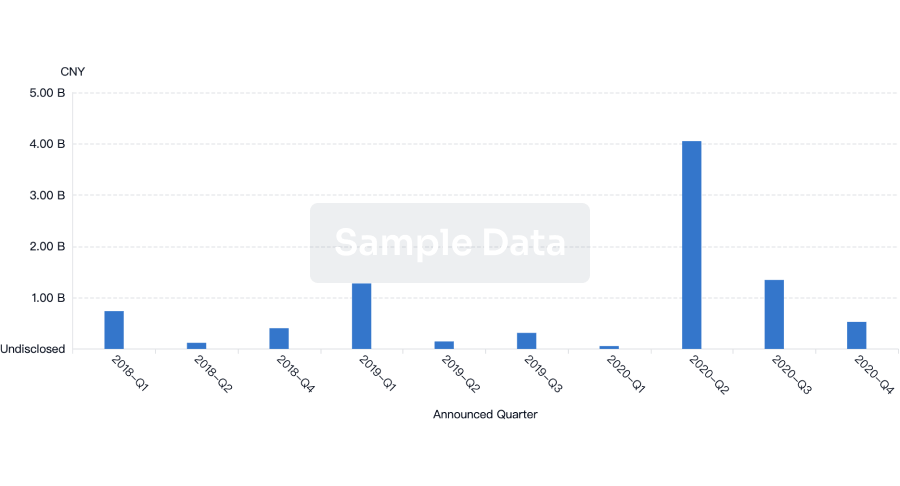
Financing
Unearth financing trends to validate and advance investment opportunities.
login
or
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free