/ CompletedNot Applicable Sweeteners and Sweetness Enhancers: Prolonged Effects on Health, Obesity and Safety
The aim of this randomised controlled trial (RCT) is to investigate if prolonged consumption of sweetener and sweetness enhancers (S&SEs) within a healthy diet approach will improve weight loss maintenance and obesity related risk factors, and affect safety markers, compared to sugar.
We hypothesize, that:
Prolonged use of S&SEs in beverages and food matrices will result in improved body weight control because S&SEs will increase palatability of the diet and thereby increase compliance to the recommendations for a healthy diet.
There will be no safety concerns using S&SEs in the long term.
Overweight/obese adults and families where at least one adult (both gender) and one child (both gender) are overweight/obese will be recruited. The majority of measurements will only be conducted in the adult population and some measurement will only be done in sub-groups. The intervention will be performed in four countries: Denmark, Greece, Spain and the Netherlands.
The goal is approximately 370 participants - 330 adults (18-65 years of age) and 40 children (6-12 years of age) - will be recruited for the study. All adult participants are first treated by a low energy diet (LED) for 2 months with the aim to reduce body weight (minimum 5% weight loss (WL)), whereas children are treated separately with a conventional weight maintenance (WM) diet, without a specific aim for absolute WL.
The participants - both adults and families - are randomized into two different diet interventions for 10 months with or without inclusion of S&SEs products (foods and drinks). For adults, this period aims at preventing weight re-gain and for children maintaining body mass index (BMI)-for-age. The participants will receive food exchange lists and will be guided by dieticians. The randomization will be stratified by age, sex and BMI. Adults (not participating with children) belonging to the same household and all members of a family will be assigned the same intervention - the randomization will here solely be based on the oldest adult in the family/household.
The adult participants are weighed at months 0, 0.5 and 1, and if needed at month 1.5. They are supervised during the WL period at months 0 and 1, and if needed at months 0.5 and 1.5, and throughout the WM period at months 2, 4, 6, 9 and 12. Children will follow a similar, but less strict time schedule (their participation is preferred but not required for all dietician meetings).
The main assessment points are the clinical investigation days (CIDs) at month 0 (baseline, start of the WL period), 2 (end of the WL period/start of randomized intervention), 6 (6 months from baseline) and 12 (1 year from baseline).
/ CompletedNot Applicable PREVention of Diabetes Through Lifestyle Intervention and Population Studies in Europe and Around the World
Type-2 diabetes is one of the fastest growing chronic diseases worldwide. This trend is mainly driven by a global increase in the prevalence of obesity. The PREVIEW study has been initiated to find out the most effective lifestyle-components (diet and physical activity) in the prevention of Type-2 diabetes. The project consists of a randomized lifestyle-intervention with the more specific aim to determine the preventative impact of a high-protein and low-GI diet in combination with moderate or high intensity physical activity compared with a moderate-protein and moderate GI diet in combination with the same activity levels on the incidence of Type-2 diabetes in predisposed, pre-diabetic children, young and older adults.
The trial will be performed in 6 EU countries (Bulgaria, Denmark, Finland, Spain, Netherlands, UK) and Australia and New Zealand.
A total of 2,500 overweight or obese adult participants (25-70 y) as well as 150 children and adolescents aged 10-18 y) will be recruited. All adult participants are first treated by a low-calorie diet for 8 weeks, with an aim to reach ≥ 8% weight reduction. Children and adolescents are treated separately with a conventional weight-reduction diet, with-out a specific aim for absolute weight loss.
The adult participants are randomized into two different diet interventions and two exercise interventions for a total of 148 weeks. This period aims at preventing Type-2 diabetes by weight-maintenance (prevention of relapse in reduced body weight) and by independent metabolic effects of diet and physical activity.
The primary endpoint of the study is the incidence of Type-2 diabetes in the adults during 3 years (156 weeks) according to diet (high protein/low-GI versus moderate protein/moderate-GI, adjusted for physical activity), based on a 75 g oral glucose tolerance test and/or HbA1c.
For children and adolescents:
Change in insulin resistance at 2 years after randomization to high protein versus moderate protein diet, measured by insulin resistance analyzed by the homeostatic model (HOMA-IR) as well as physiological improvement of health with respect to pre-diabetic characteristics.
Our hypothesis is that a high-protein, low-GI diet will be superior in preventing type-2 diabetes, compared with a moderate protein, moderate GI diet, and that high-intensity physical activity will be superior compared to moderate-intensity physical activity.
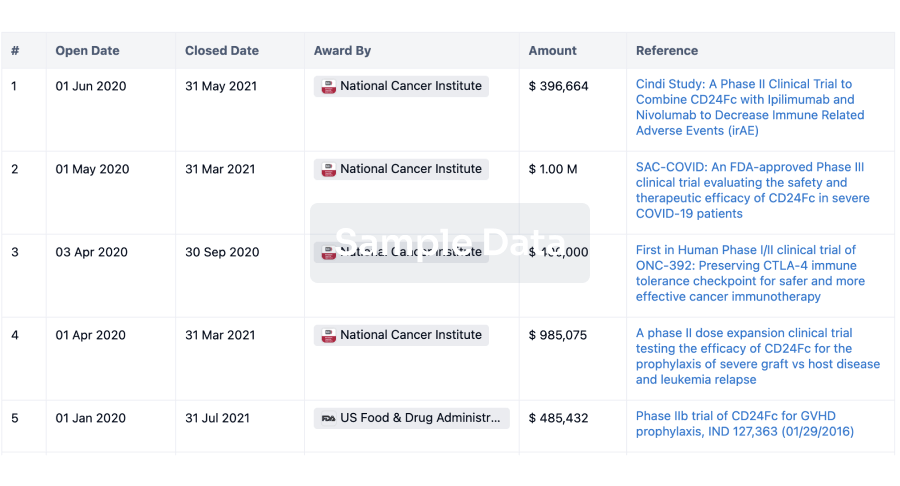
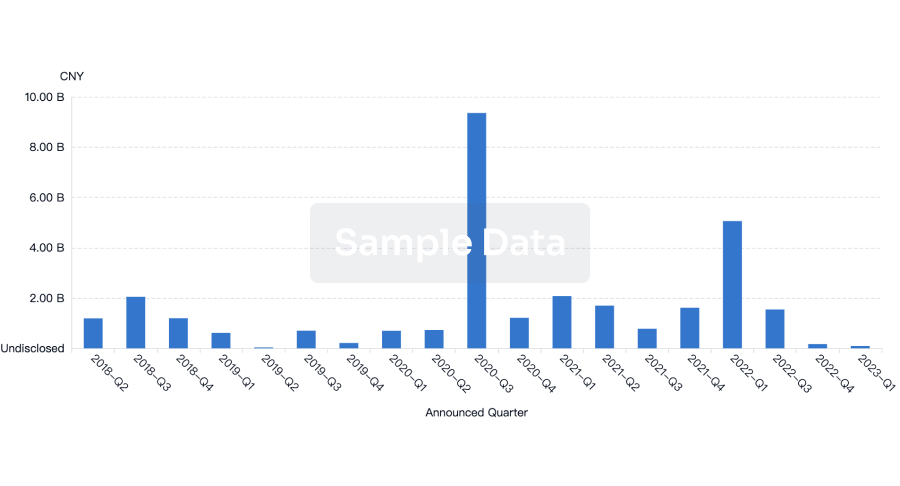
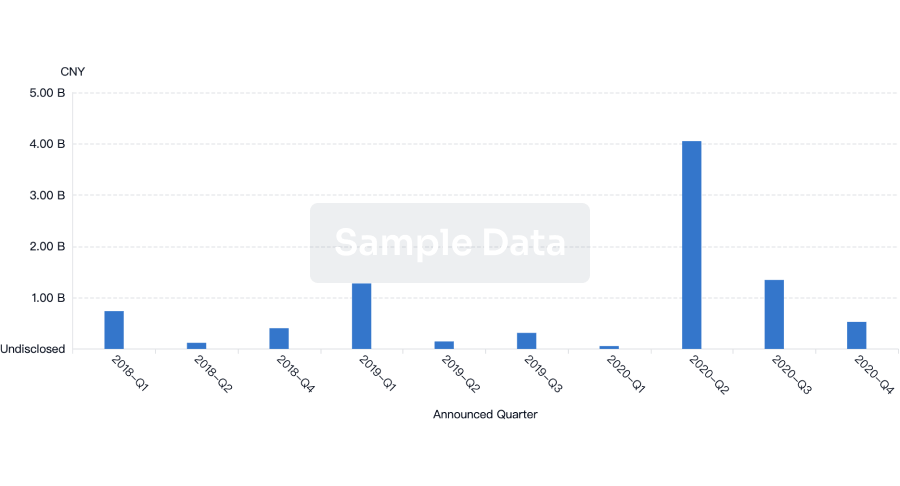
100 Clinical Results associated with NetUnion SARL
0 Patents (Medical) associated with NetUnion SARL
100 Deals associated with NetUnion SARL
100 Translational Medicine associated with NetUnion SARL