/ Not yet recruitingPhase 1 A Phase I, Randomized, Crossover, Double-blind, Pharmacokinetic Study of Berberine Released From Cyclodextrin in Healthy Volunteers
In this study, we will evaluate the relative bioavailability of Berberine (BB) from capsules containing Indian Barberry (Berberis aristate DC.) Bark and Root Extract in the blood plasma of healthy subjects after oral administration of:
A. Capsules containing Berberine and GCD (BBA Berberine MetX™ Ultra Absorption, 250 mg) B. Capsules containing Berberine (BB, Berberine MetX™, 500 mg) - (reference product).
Dissolution and Pharmacokinetic of Ginsenosides Released From Cyclodextrin Based Chewable Tablets: a Comparative, Randomized, Crossover, Open-label Study in Healthy Human Subjects.
This study will evaluate the relative bioavailability of ginsenosides Rg5, Rk1, and Ck of Red ginseng HRG80 preparations containing gamma-cyclodextrin (GCD) in the blood plasma of healthy subjects after oral administration of two different formulations of HRG80:
A. Capsules containing red ginseng preparation HRG80 (reference product) B. Chewable tablets containing red ginseng preparation HRG80 and GCD (modified product).
Dissolution testing measures the rate and extend water solubility of ginsenosides from the reference (A) and the modified (B) products. The difference of in vitro dissolution profiles between the reference (A) and modified (B) products will be assessed.
/ Unknown statusPhase 2/3 Effect of Kan-Jang® Supplementation in Patients Diagnosed With COVID-19: A Randomized, Quadruple-blind, Placebo-controlled Trial
The complexity of COVID-19 suggests a potential need for a range of therapies, including antiviral agents, immunostimulants, immunosuppressants, adaptogens, and anticoagulants. In this context, implementation of polyvalency drugs, which exhibit a wide range of biological activities and multitarget effects that is common for herbal medicines and specifically for Kan Jang, the fixed combination of Andrographis paniculata (Burm. F.) Wall. ex. Nees and Eleutherococcus senticosus (Rupr. & Maxim.) Maxim which are known to exhibit antiviral, immunomodulatory, and anti-inflammatory effects and clinical efficacy in the respiratory tract of patients with infectious diseases. The purpose of this study is to provide scientific evidence on the effectiveness of Kan Jang for the treatment of mild COVID-19. We hypothesize that Kan Jang will have superior efficacy in amelioration COVID symptoms compared to placebo with a comparable safety profile to placebo. We hypothesize that Kan Jang will increase patients' recovery rate and decrease the duration of illness.
The objective of the study is to assess the efficacy and tolerability of adjuvant treatment with Kan Jang for alleviating the severity of inflammatory symptoms (headache, loss of smell, gustatory dysfunction, rhinorrhea, nasal congestions, cough, sore throat, asthenia, myalgia, and fever) and shortening of their duration in mild COVID-19 patients.
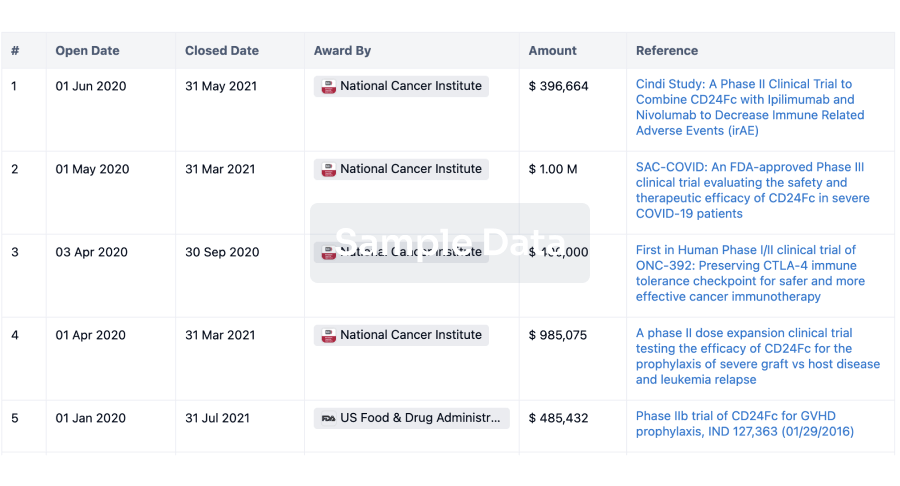
100 Clinical Results associated with Phytomed AB
0 Patents (Medical) associated with Phytomed AB
100 Deals associated with Phytomed AB
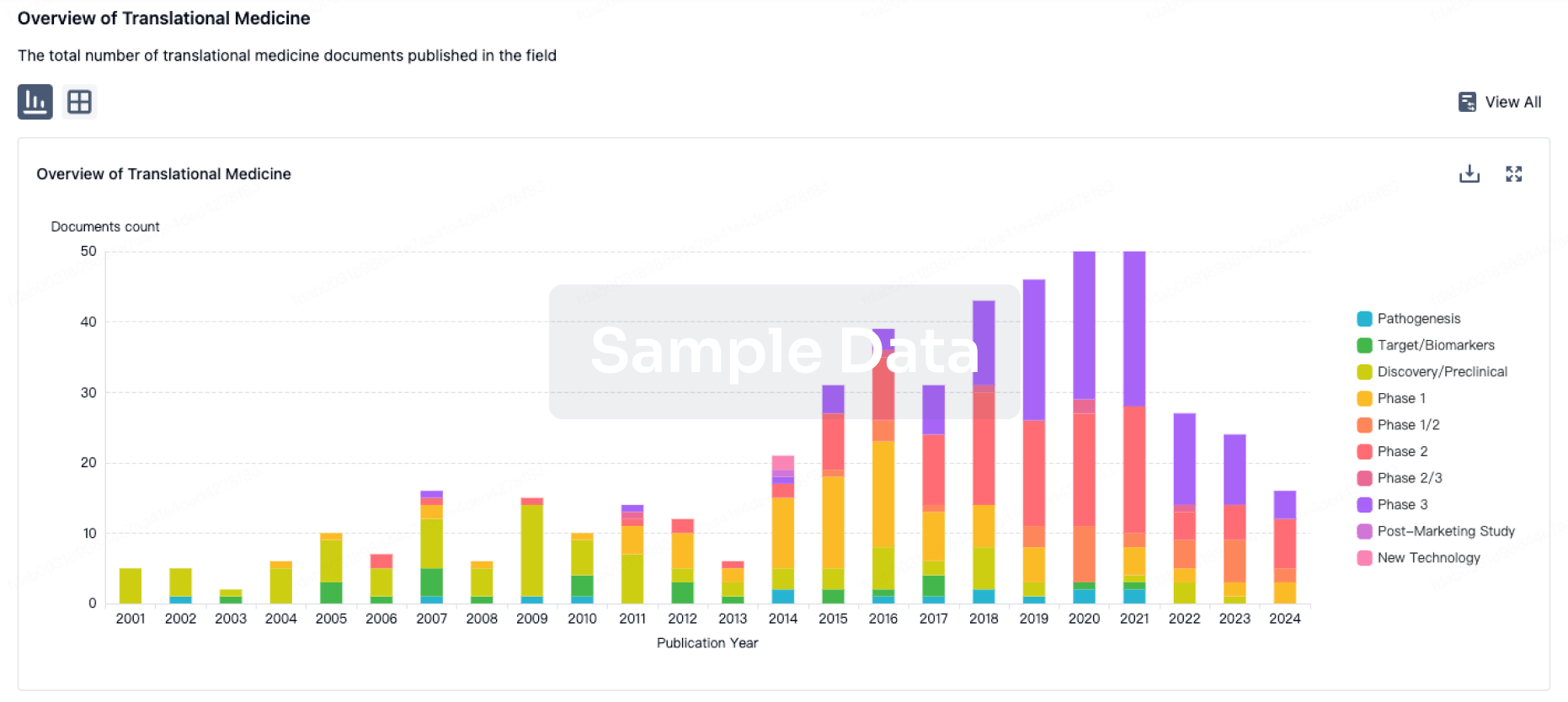
100 Translational Medicine associated with Phytomed AB