100 Clinical Results associated with Innovative Biologics, Inc.
0 Patents (Medical) associated with Innovative Biologics, Inc.
01 Oct 2017·International Journal of PharmaceuticsQ2 · MEDICINE
Inhibition of Clostridium perfringens epsilon toxin by β-cyclodextrin derivatives
Q2 · MEDICINE
Article
Author: Karginov, Vladimir A ; Karginov, Andrei V ; Robinson, Tanisha M ; Jicsinszky, Laszlo
Clostridium perfringens epsilon toxin (ETX) is considered as one of the most dangerous potential biological weapons. The goal of this work was to identify inhibitors of ETX using a novel approach for the inactivation of pore-forming toxins. The approach is based on the blocking of the target pore with molecules having the same symmetry as the pore itself. About 200 various β-cyclodextrin derivatives were screened for inhibitors of ETX activity using a colorimetric cell viability assay. Several compounds with dose-dependent activities at low micromolar concentrations have been identified. The same compounds were also able to inhibit lethal toxin of Bacillus anthracis.
01 Oct 2013·Current opinion in pharmacology
Cyclodextrin derivatives as anti-infectives.
Review
Author: Karginov, Vladimir A
Cyclodextrin derivatives can be utilized as anti-infectives with pore-forming proteins as the targets. The highly efficient selection of potent inhibitors was achieved because per-substituted cyclodextrins have the same symmetry as the target pores. Inhibitors of several bacterial toxins produced by Bacillus anthracis, Staphylococcus aureus, Clostridium perfringens, Clostridium botulinum, and Clostridium difficile were identified from a library of ∼200 CD derivatives. It was demonstrated that multi-targeted inhibitors can be found using this approach and could be utilized for the development of broad-spectrum drugs against various pathogens.
01 Jul 2011·Antimicrobial Agents and ChemotherapyQ2 · MEDICINE
Symmetry Requirements for Effective Blocking of Pore-Forming Toxins: Comparative Study with α-, β-, and γ-Cyclodextrin Derivatives
Q2 · MEDICINE
Article
Author: Bezrukov, Sergey M. ; Robinson, Tanisha M. ; Mourtzis, Nikolaos ; Nestorovich, Ekaterina M. ; Jicsinszky, Laszlo ; Yohannes, Adiamseged ; Karginov, Vladimir A. ; Aggelidou, Crysie ; Yannakopoulou, Konstantina
ABSTRACT
We compared the abilities of structurally related cationic cyclodextrins to inhibit
Bacillus anthracis
lethal toxin and
Staphylococcus aureus
α-hemolysin. We found that both β- and γ-cyclodextrin derivatives effectively inhibited anthrax toxin action by blocking the transmembrane oligomeric pores formed by the protective antigen (PA) subunit of the toxin, whereas α-cyclodextrins were ineffective. In contrast, α-hemolysin was selectively blocked only by β-cyclodextrin derivatives, demonstrating that both symmetry and size of the inhibitor and the pore are important.
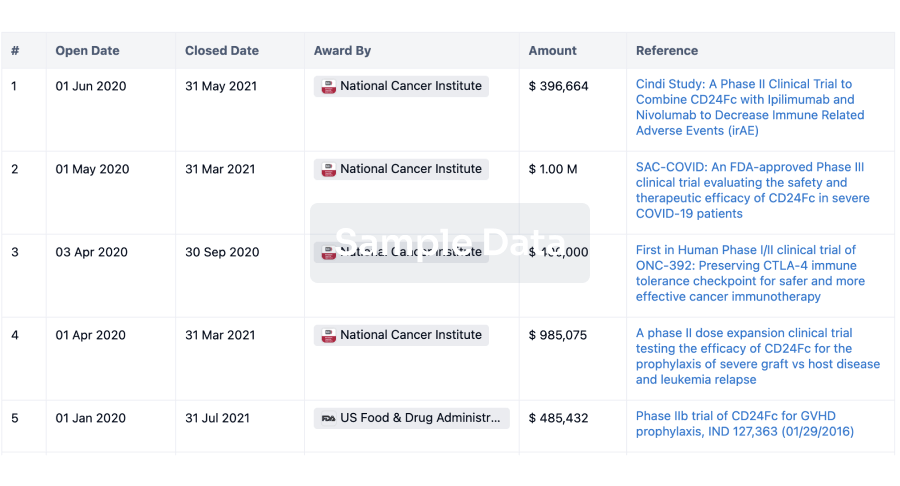
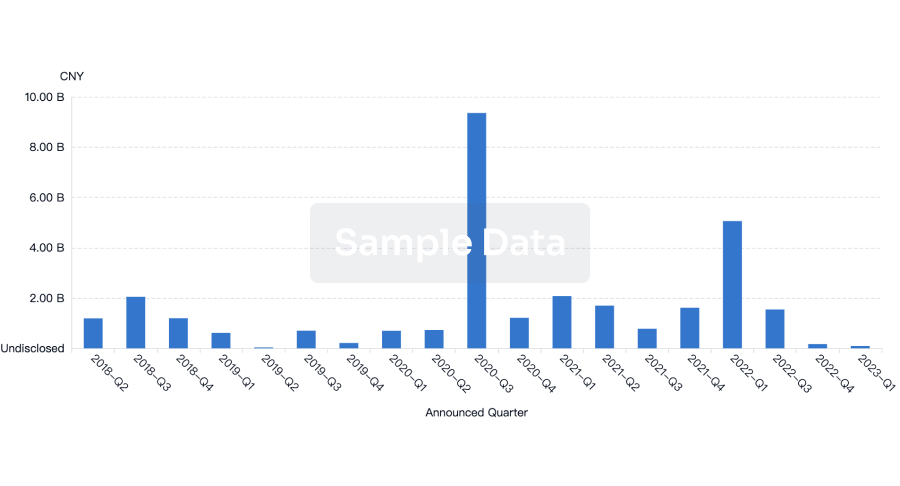
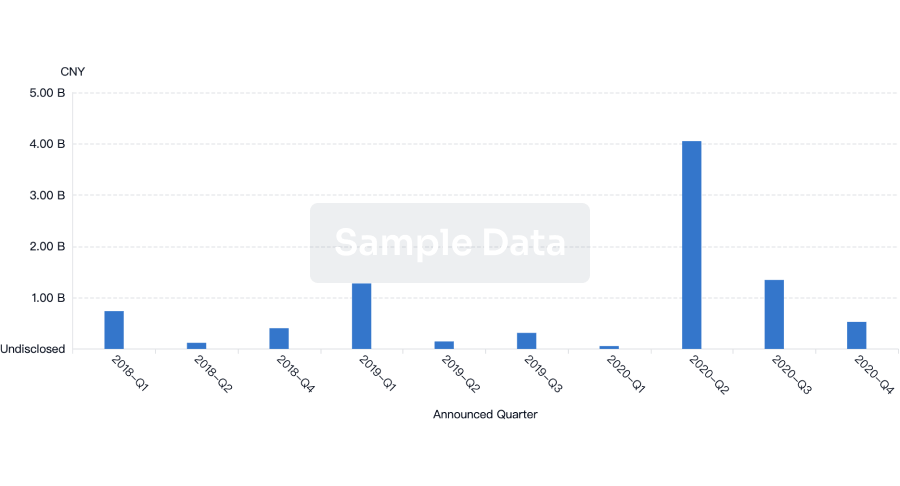
100 Deals associated with Innovative Biologics, Inc.
100 Translational Medicine associated with Innovative Biologics, Inc.