A Pilot, Randomized, Open-label Trial to Determine the Feasibility, Safety, Efficacy, and Pharmacokinetics of Nebulized HCQ01 for the Treatment of Patients With COVID-19 and Cancer
This is a pilot, randomized, single-center, parallel group, open-label controlled study to evaluate the feasibility, safety, efficacy, and pharmacokinetics of nebulized HCQ01 plus Standard of Care (SOC) versus SOC alone in hospitalized cancer patients with COVID-19. King Hussein Cancer Center (KHCC) is the study sponsor, and the study will be conducted at KHCC COVID-19 wards.
Approximately 28 cancer patients, ≥18 years of age with a confirmed SARS-CoV-2 infection, will be enrolled and randomized 1:1 to the treatment and control arms where they will receive ten doses of Hydroxychloroquine solution via nebulizer in addition to SOC or the control arm where treatment will follow KHCC SOC.
Randomized, Two-way, Two-period, Single Oral Dose, Open-label, Crossover, Bioequivalence Study to Compare Darfen 400, 400 mg Ibuprofen Coated Tablets (512 mg Ibuprofen Sodium Dihydrate) Versus Nurofen® Forte Express, 400 mg Ibuprofen Coated Tablets (512 mg Ibuprofen Sodium Dihydrate) in Healthy Adult Male and Female Subjects Under Fasting Conditions
This study is a comparative bioavailability study performed to assess bioequivalence between Test medicinal product (Darfen 400, 400 mg ibuprofen coated tablets [512 mg ibuprofen sodium dihydrate], manufactured by PrJSC "Pharmaceutical firm "Darnitsa" [Ukraine]) and Reference medicinal product (marketed medicinal product Nurofen® Forte Express, 400 mg ibuprofen coated tablets [512 mg ibuprofen sodium dihydrate], manufactured by Reckitt Benckiser [Poland] S.A.) in healthy volunteers.
/ Unknown statusPhase 2IIT A Pilot, Randomized, Open-label Trial to Determine the Feasibility, Safety, Efficacy, and Pharmacokinetics of Nebulized HCQ01for the Treatment of Patients With COVID-19
This is a pilot, randomized, single-center, parallel group, open-label controlled study to evaluate the feasibility, safety, efficacy, and pharmacokinetics of nebulized HCQ01 plus Standard of Care (SOC) versus SOC alone in hospitalized COVID-19 patients. The Jordanian Ministry of Health (MOH) is the study sponsor, and the study will be conducted at MOH COVID-19 hospitals.
Approximately 110 patients, ≥18 years of age with a confirmed SARS-CoV-2 infection, will be enrolled and randomized 1:1 to the treatment and control arms where they will receive ten doses of Hydroxychloroquine solution via nebulizer in addition to SOC or the control arm where treatment will follow the MOH SOC.
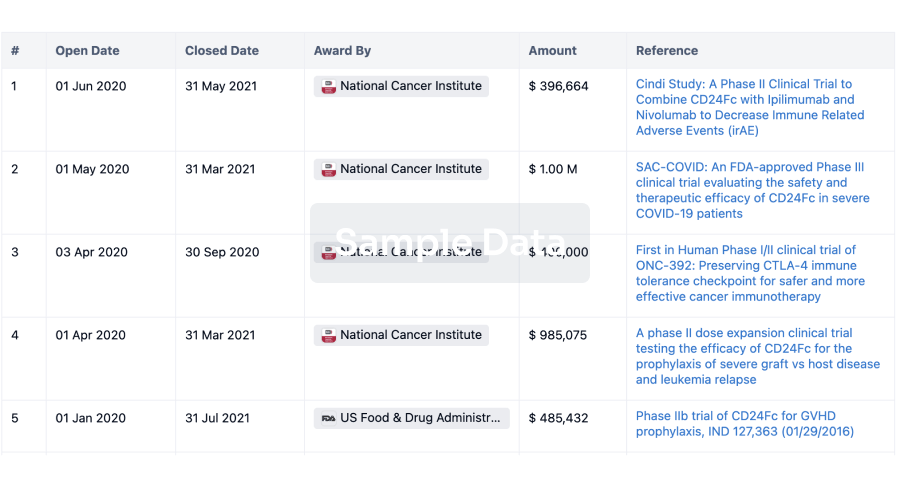
100 Clinical Results associated with ACDIMA Center for Bioequivalence and Pharmaceutical Studies (ACDIMA BioCenter)
0 Patents (Medical) associated with ACDIMA Center for Bioequivalence and Pharmaceutical Studies (ACDIMA BioCenter)
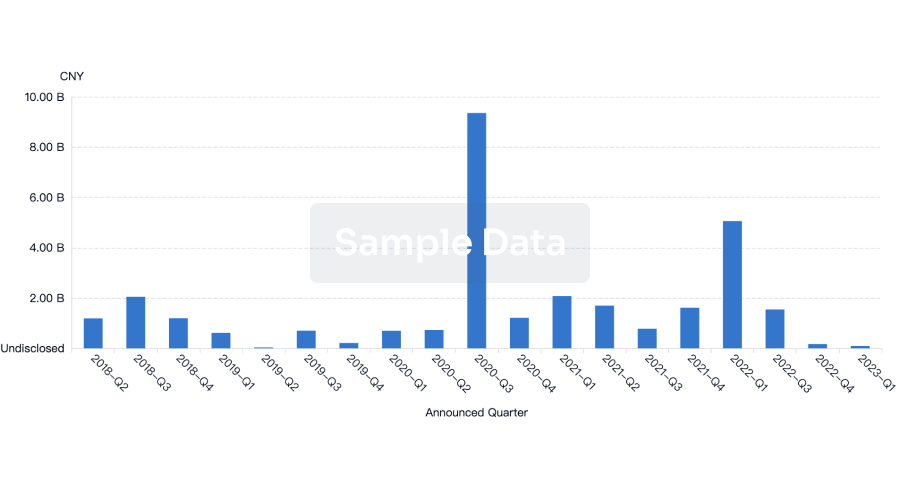
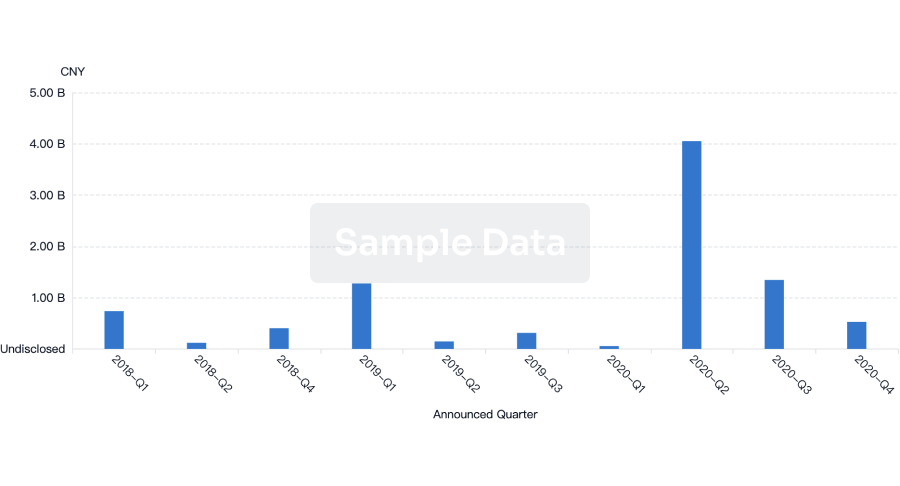
100 Deals associated with ACDIMA Center for Bioequivalence and Pharmaceutical Studies (ACDIMA BioCenter)
100 Translational Medicine associated with ACDIMA Center for Bioequivalence and Pharmaceutical Studies (ACDIMA BioCenter)