Request Demo
Last update 08 May 2025
SCAF1
Last update 08 May 2025
Basic Info
Synonyms FLJ00034, SCAF, SCAF1 + [9] |
Introduction May function in pre-mRNA splicing. |
Related
100 Clinical Results associated with SCAF1
Login to view more data
100 Translational Medicine associated with SCAF1
Login to view more data
0 Patents (Medical) associated with SCAF1
Login to view more data
131
Literatures (Medical) associated with SCAF101 Mar 2025·Journal for ImmunoTherapy of Cancer
PLS-α-GalCer: a novel targeted glycolipid therapy for solid tumors
Article
Author: Kularatne, Ruvanthi N ; Tiwary, Shweta ; Berzofsky, Jay A ; Skoczen, Sarah L ; Burks, Julian ; Stevens, David M ; Stern, Stephan T
08 Feb 2025·Nucleic Acids Research
Phenotypic screens identify SCAF1 as critical activator of RNAPII elongation and global transcription
Article
Author: Eilers, Martin ; Wolf, Elmar ; Lamer, Stephanie ; Eilers, Ursula ; Schülein-Völk, Christina ; Wenzel, Julia ; Erhard, Florian ; Bhandare, Pranjali ; Rummel, Teresa ; Hofstetter, Julia ; Narain, Ashwin ; Schlosser, Andreas
01 Feb 2025·Journal of Investigative Medicine
Plasma from type 1 diabetes patients promotes pro-atherogenic cholesterol transport in human macrophages
Article
Author: Reiss, Allison B ; Kasselman, Lora J ; Voloshyna, Iryna ; Srivastava, Ankita ; Levine, Robert L ; Renna, Heather A ; Mejia-Corletto, Jorge ; De Leon, Joshua ; Accacha, Siham
Analysis
Perform a panoramic analysis of this field.
login
or
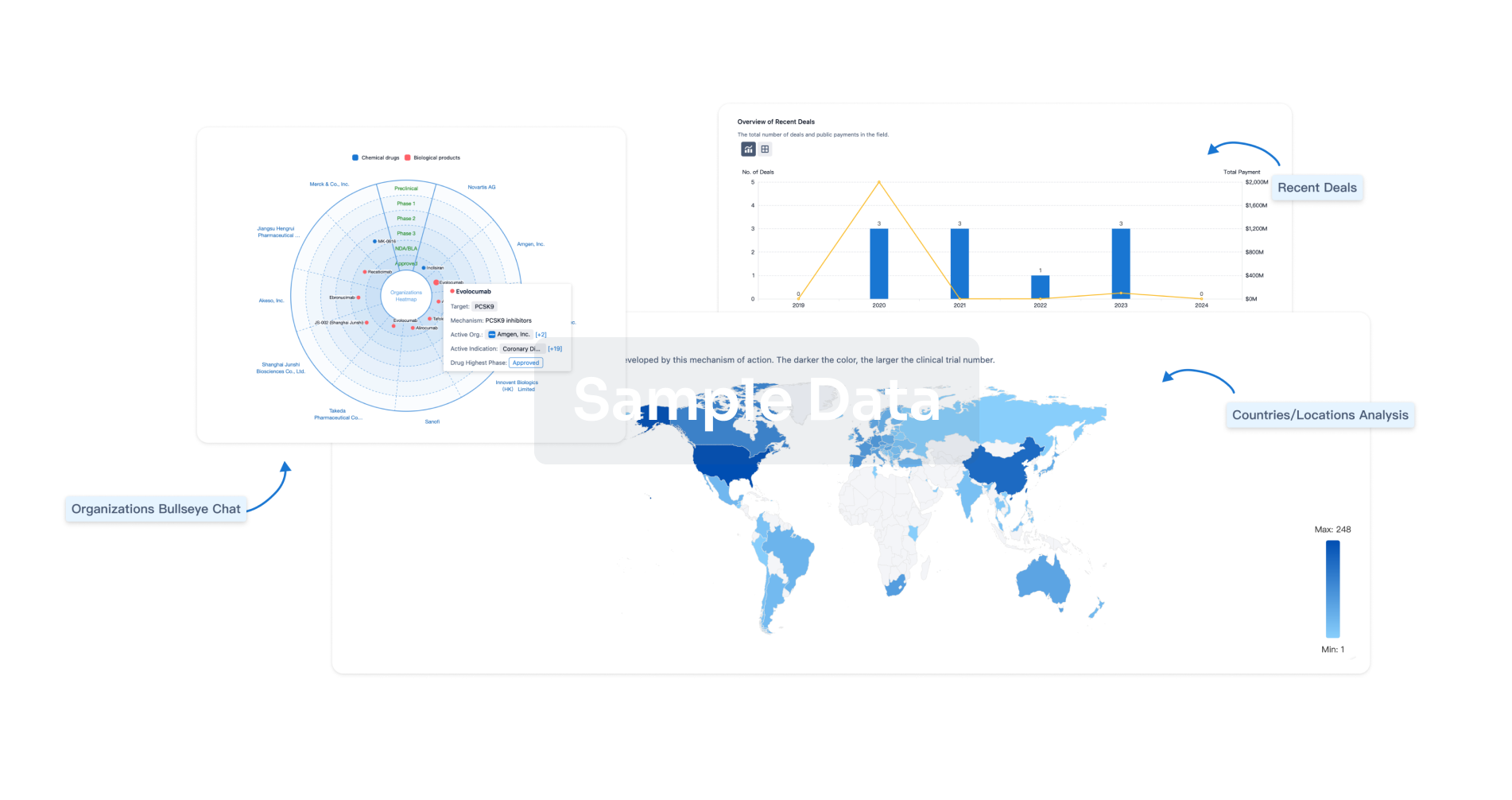
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free