Request Demo
Last update 23 Jan 2025
CHI3L2
Last update 23 Jan 2025
Basic Info
Synonyms CHI3L2, chitinase 3 like 2, Chitinase-3-like protein 2 + [3] |
Introduction Lectin that binds chitooligosaccharides and other glycans with high affinity, but not heparin. Has no chitinase activity. |
Related
100 Clinical Results associated with CHI3L2
Login to view more data
100 Translational Medicine associated with CHI3L2
Login to view more data
0 Patents (Medical) associated with CHI3L2
Login to view more data
135
Literatures (Medical) associated with CHI3L206 Aug 2024·Current Medicinal Chemistry
Identification of Key Genes and Signaling Pathways in Entrectinibresistant
Non-small Cell Lung Cancer Using Bioinformatic Analysis
and Experimental Verification
Article
Author: Zhao, Xin ; Li, Dongbing ; Zha, Wangjian ; Chen, Xuesong
01 Aug 2024·Neuroscience
Alcohol drinking modified the effect of plasma YKL-40 levels on stroke-specific mortality of acute ischemic stroke
Article
Author: Che, Bizhong ; Zheng, Xiaowei ; Zhang, Kaixin ; Zhang, Yonghong ; Zhu, Zhengbao ; Wang, Ziyi ; Xu, Tian ; Yang, Pinni ; Lu, Yaling ; Zhong, Chongke
01 Jul 2024·FEBS Open Bio
Exploring the role of CD8 + T cells in clear renal cell carcinoma metastasis
Article
Author: Meng, Lingzhang ; Ling, Caixia ; Luo, Yanhong ; Shen, Jiajia ; Liang, Zhengfang ; Qin, Yujuan ; Huang, Shaoang ; Chen, Yuanhong ; Lin, Wenxian
Analysis
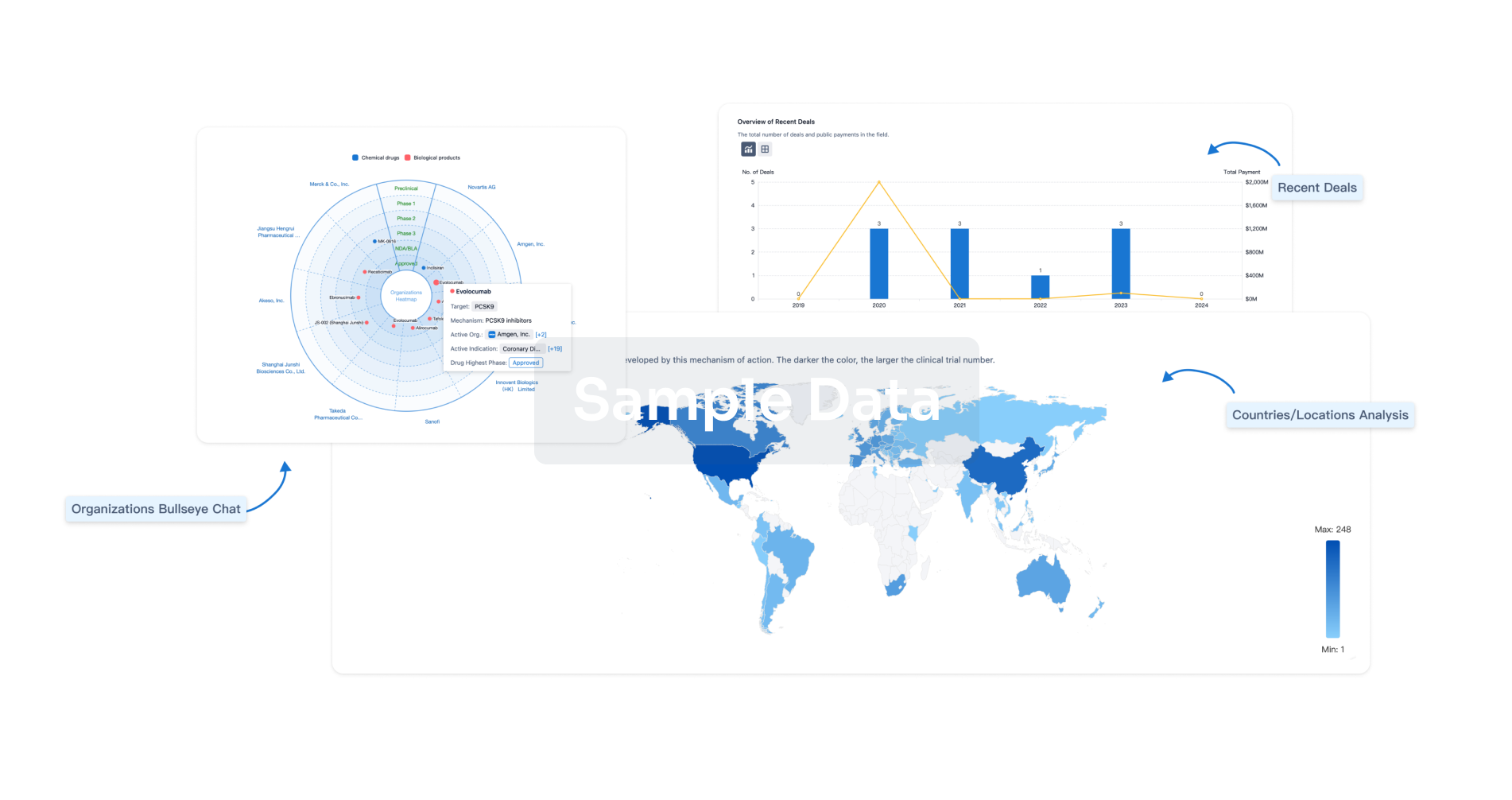
Perform a panoramic analysis of this field.
login
or
Chat with Hiro
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free