Request Demo
Last update 08 May 2025
CEP170
Last update 08 May 2025
Basic Info
Related
100 Clinical Results associated with CEP170
Login to view more data
100 Translational Medicine associated with CEP170
Login to view more data
0 Patents (Medical) associated with CEP170
Login to view more data
64
Literatures (Medical) associated with CEP17001 Jun 2025·Journal of Molecular Biology
Cryo-EM of AKAP350/AKAP9 Reveals Fibrillar Clusters and an Association With DNA
Article
Author: Raught, Brian ; Liu, Alex C H ; Keszei, Alexander F A ; Goldenring, James R ; Dai, David L ; Zhang, Xu ; Kolobova, Elena ; St-Germain, Jonathan ; Mazhab-Jafari, Mohammad T ; Hasan, S M Naimul
01 Jan 2025·International Immunopharmacology
METTL3-mediated CEP170 m6A modifications in spindle orientation and esophageal cancer cell proliferation
Article
Author: Luan, Yi ; Ren, Kaidi ; Yan, Dan ; Wu, Kai ; Zhang, Xiang ; Yin, Fanxiang ; He, Hongbo ; Qin, Bo ; Xia, Chaoyuan ; Zhao, Xian ; Yang, Yang ; Jue, Bolin
01 Oct 2024·Modern Pathology
RNA Sequencing May Call CEP170::RAD51B Fusion Because of Alignment Artifacts
Letter
Author: Zhou, Peng ; Sun, Yi ; Liang, Qingchun ; Lei, Cheng
1
News (Medical) associated with CEP17014 Jul 2022
Most cancer research and available anticancer drugs focus on the impact of DNA and protein alterations that contribute to cancer; however, it is now understood that RNA molecules can also both positively and negatively impact the development of cancer. Researchers now describe how RNA molecules promote the development of melanoma metastasis by impacting anti-tumor microRNA.
Changes in DNA can lead to the development and progression of cancer. DNA serves as a template for an intermediary molecule called RNA that, in turn, codes for proteins that control all cellular processes. Most cancer research and available anticancer drugs focus on the impact of DNA and protein alterations that contribute to cancer; however, it is now understood that RNA molecules can also both positively and negatively impact the development of cancer. In a new article published in Cancer Research, a journal of the American Association for Cancer Research, Moffitt Cancer Center researchers describe how RNA molecules promote the development of melanoma metastasis by impacting anti-tumor microRNA.
MicroRNA (miRNA) molecules are small segments of nonprotein coding RNA that can silence other protein-coding RNA molecules and regulate the production of proteins. When the activity of miRNA molecules is perturbed, diseases such as cancer can develop. Recently, it has been shown that this regulation also works in the opposite direction, where protein coding RNAs act as "sponges" to bind to miRNA molecules and block their function. RNAs affecting the function of miRNAs are called competitive endogenous RNA (ceRNA) and are thought to play an important role in cancer development independent of their protein-coding activity.
The existence of ceRNA has been known since approximately 2010, but its impact on cancer development is not well understood. Since DNA copy number alterations impact cancer, Moffitt researchers wanted to determine whether these DNA alterations can drive cancer development through ceRNAs.
The team analyzed chromosome alterations and discovered that gains in chromosome segment 1q were very common among a panel of metastatic melanoma cases. A more in-depth analysis revealed that three key genes called CEP170, NUCKS1andZC3H11A present on chromosome 1q are amplified in metastatic melanoma cases and associated with disease progression.
Given the potential clinical implications of these alterations, the researchers wanted to understand the molecular contributions of CEP170, NUCKS1andZC3H11Ato melanoma development. They performed a series of laboratory experiments and discovered that the RNA sequences of CEP170, NUCKS1andZC3H11A promote cell growth, migration and invasion in melanoma cell lines, and stimulated metastasis growth in mouse models of melanoma, independent of their protein-coding activity. Mechanistically, the researchers discovered that the RNA sequences of the three genes act as ceRNAs that sponge miRNA molecules that function to inhibit tumor growth and development. Therefore, by "soaking up" the miRNA molecules and their blocking antitumor activity, the ceRNA molecules drive tumor growth and metastasis. Importantly, the researchers discovered that copy number alterations of CEP170, NUCKS1andZC3H11A were present in other tumor types, including breast, colon, liver and lung cancer, suggesting that these alterations may be important for other cancer types as well.
These results will likely change the widely held view that the key to cancer primarily relies on the structure and function of proteins and opens avenues for new investigations into a biological area that is only beginning to be studied.
"Our study challenges the notion that somatic copy number alterations promote cancer predominantly through their encoded proteins and establishes ceRNAs as potent drivers underlying the oncogenicity of somatic copy number alterations," said Florian Karreth, Ph.D., study author and assistant member of the Molecular Oncology Department.
This study was supported by the National Institutes of Health (R03CA227349, R01CA259046 and P30CA076292) and the Melanoma Research Alliance (500655).
AACR
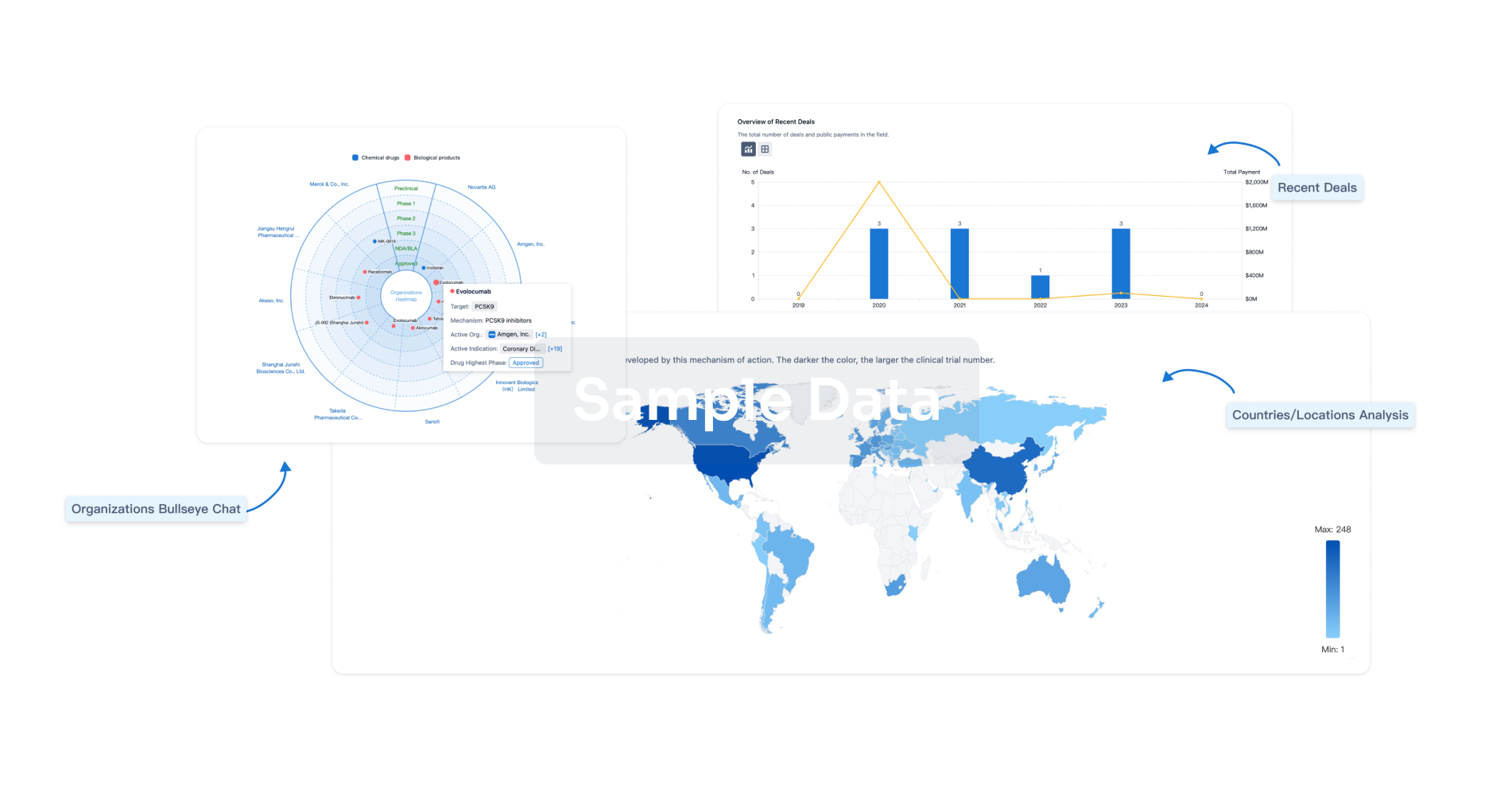
Analysis
Perform a panoramic analysis of this field.
login
or
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free