Request Demo
Last update 08 May 2025
C19orf48
Last update 08 May 2025
Basic Info
Synonyms BC006151, C19orf48, chromosome 19 open reading frame 48 + [3] |
Introduction- |
Related
100 Clinical Results associated with C19orf48
Login to view more data
100 Translational Medicine associated with C19orf48
Login to view more data
0 Patents (Medical) associated with C19orf48
Login to view more data
590
Literatures (Medical) associated with C19orf4803 May 2025·Journal of Biomolecular Structure and Dynamics
Comprehending conformational changes in EmrE, multidrug transporter at different pH: insights from molecular dynamics simulations
Article
Author: Kaur, Manpreet ; Arya, Preeti ; Singh, Balvinder ; Chosyang, Stanzin
01 Apr 2025·Journal of Molecular Biology
Conversion of Human Multidrug Transporter P-glycoprotein (ABCB1) from Drug Efflux to Uptake Pump: Evidence for a Switch Region Modulating the Direction of Substrate Transport
Article
Author: Ranganathan, Nandhini ; Ahmed, Shafaq ; Durell, Stewart R ; Sajid, Andaleeb ; Ambudkar, Suresh V ; Guha, Rajan ; Murakami, Megumi
01 Mar 2025·Journal of Global Antimicrobial Resistance
Nationwide phylogenomic surveillance of Mycobacterium tuberculosis in Mexico reveals pathogenic and drug resistant signatures of the prevailing L4 sublineage
Article
Author: Martinez-Urtaza, Jaime ; Garcia-Ulloa, Manuel ; Martinez-Guarneros, Armando ; Vazquez-Chacon, Carlos A ; Alvarez-Maya, Ikuri
Analysis
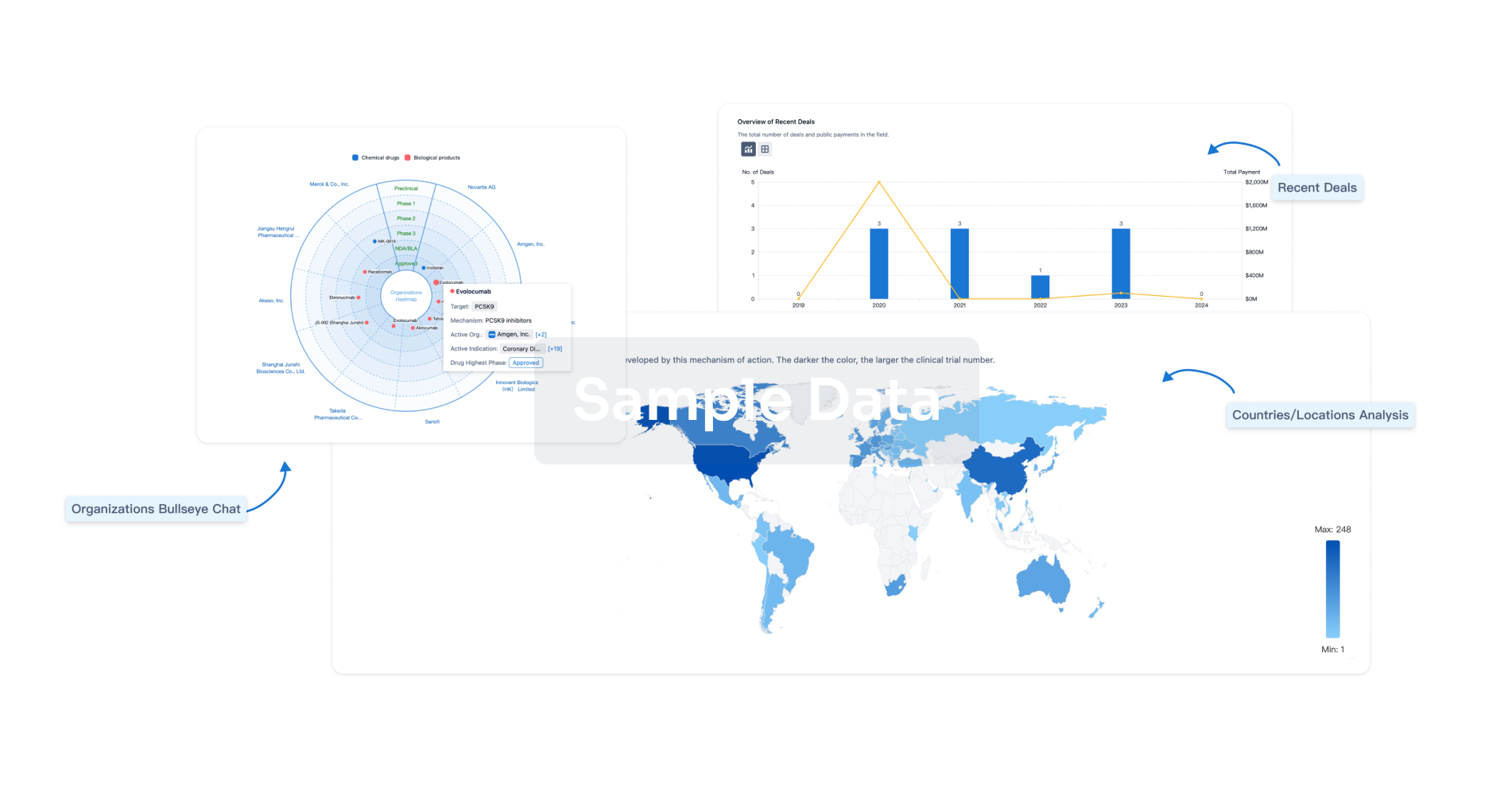
Perform a panoramic analysis of this field.
login
or
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free