Request Demo
Last update 08 May 2025
COL11A1
Last update 08 May 2025
Basic Info
Synonyms CO11A1, COL11A1, COLL6 + [4] |
Introduction May play an important role in fibrillogenesis by controlling lateral growth of collagen II fibrils. |
Related
100 Clinical Results associated with COL11A1
Login to view more data
100 Translational Medicine associated with COL11A1
Login to view more data
0 Patents (Medical) associated with COL11A1
Login to view more data
643
Literatures (Medical) associated with COL11A101 Jun 2025·Poultry Science
Spatial transcriptomic differences in the breast muscle of grower broilers at 21 and 28 days of age
Article
Author: Bowker, Brian ; Zhuang, Hong ; Gudidoddi, Seshidhar Reddy ; Goo, Doyun ; Choppa, Venkata Sesha Reddy ; Han, Gippeum ; Shakeri, Majid ; Lee, Jihwan ; Kong, Byungwhi ; Choi, Janghan ; Kim, Woo Kyun
01 Apr 2025·Pharmacological Research
Knowledge of the genetics of human pain gained over the last decade from next-generation sequencing
Review
Author: Lötsch, Jörn ; Kringel, Dario
01 Apr 2025·Canadian Journal of Ophthalmology
Heterozygous COL11A1 mutation associated with congenital cataract, lens subluxation, and zonular loss
Article
Author: Lee, Gabriela G. ; Panneerselvam, Sugi ; Chang, Ta Chen Peter ; Lee, Gabriela G
1
News (Medical) associated with COL11A103 May 2024
St. Jude found 156 potential targets for chimeric antigen receptor (CAR) T--cell immunotherapy. Explore the discovery's promise to improve cancer therapies.
Targeting anti-cancer therapy to affect cancer cells but not healthy cells is challenging. For chimeric antigen receptor (CAR) T-cell immunotherapy, where a patient's own immune cells are re-engineered to attack cancer cells, many solid and brain cancers lack an effective target. St. Jude Children's Research Hospital scientists have identified 156 potential targets through a comprehensive analysis paired with an experimental validation in vivo. The findings were published today in Nature Communications.
"We discovered targets for cancer immunotherapy, which hopefully can be translated in the future into curative approaches," said co-corresponding author Stephen Gottschalk, MD, St. Jude Department of Bone Marrow Transplantation and Cellular Therapy chair. "We've given the field a large set of potential targets and validated at least one, COL11A1."
While COL11A1 was one of the 156 targets identified that the researchers validated in mouse models, others showed promise in cell lines such as anti-fibronectin CAR T cells. Most targets have yet to be tested but are publicly available for other researchers to pursue.
"In addition to validating the selected targets, we have built a data resource for the community," said co-corresponding author Jinghui Zhang, PhD, St. Jude Department of Computational Biology. "The final list is available in a web portal. Others can evaluate the evidence and pursue any of these targets."
The portal, named SCE-Miner, is freely available on the St. Jude Cloud platform. External researchers can access the data and use the internal analysis tools to fuel their own research.
"Our portal empowers the users to explore these targets and the underlying data," Gottschalk said. "Other scientists can say, 'Is this target present in a particular pediatric cancer subtype?' They can access that information in the Cloud and do their own analysis."
Fishing for CAR T-cell targets
The researchers found COL11A1 and the other targets by comprehensively analyzing the subparts of genes called exons. When a gene is transcribed to RNA, only exonic regions are retained to form a mature product, which will be used as a template for protein translation. The researchers looked at whole transcriptome sequencing data of 1,532 pediatric tumor samples with 7,460 normal tissue samples, finding which exons were more highly selective or uniquely expressed in cancer cells than normal cells. Such cancer-specific exons can potentially be targeted by CAR T cells without causing harm to healthy cells. The final target list also includes several targets identified in previous analyses and are being pursued clinically, increasing confidence in the group's analytical approaches.
The St. Jude method differs in several ways compared to previous CAR target searches. The sample size was much larger, and the study involved all major cancer types. Therefore, the candidate pool was expanded. To ensure the inclusion of all relevant candidates to CAR T targets, the researchers focused on proteins existing inside the membrane, a traditional approach, and those sitting on top of the membrane in a place called the extracellular matrix, which was unusual.
"Normally, groups looking for new CAR targets looked only at membrane-associated proteins," Gottschalk said. "But one of our targets told us that we needed to broaden our criteria to include proteins of the extracellular matrix. Most of those proteins are not anchored to the cell, but it turns out they stick to the cell surface. When the CAR comes, it can still recognize that protein and kill the cancer cell."
The researchers also found that some of these genes have different exons expressed inside them, resulting in a different version of a protein called an isoform. Think of a gene as a movie; an isoform would be like a director's cut, with different scenes cut, extended or inserted while maintaining major similarities to the original. Cancer cells are prone to alternative splicing of exons, which creates isoforms, which the St. Jude analysis detected in a way that had been difficult in earlier screens.
"We used a more straightforward and robust analysis than prior approaches," Zhang said. "We could see if a subset or all set of exons within a gene were differentiated between cancer and normal cells, which allowed us to evaluate cancer-specificity at the isoform versus gene level."
Immunotherapy
Analysis
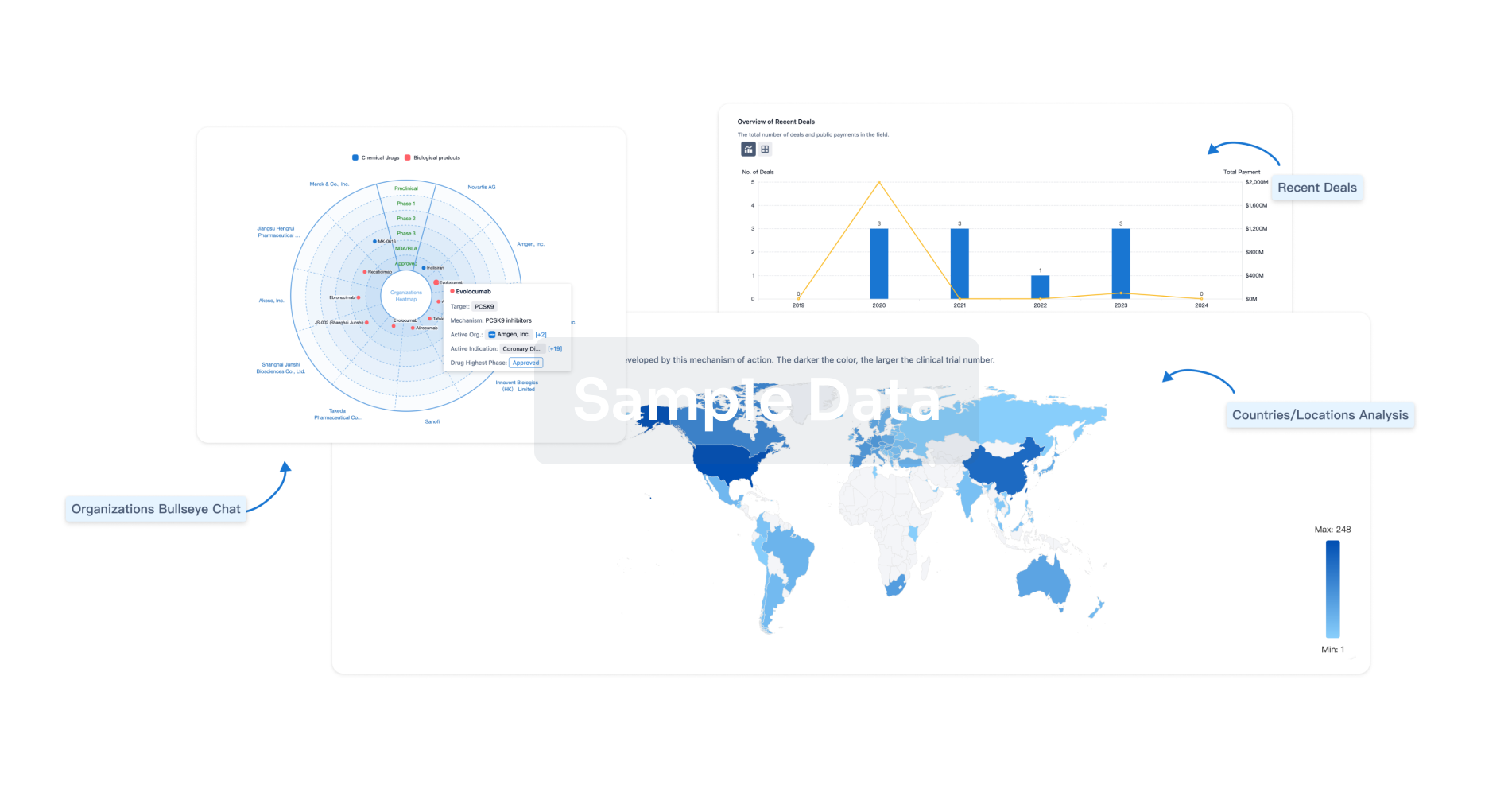
Perform a panoramic analysis of this field.
login
or
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free