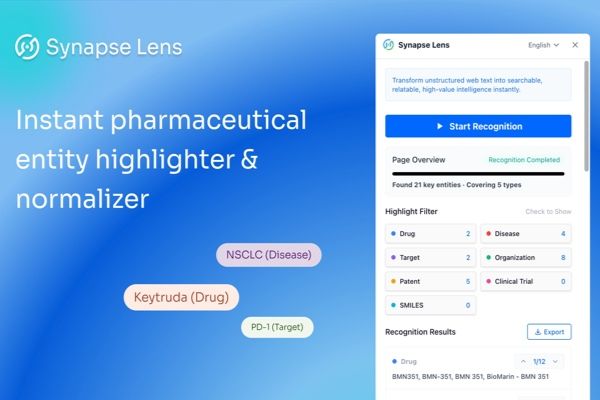
May 2025 Patent Highlights
1,Vertex continues to focus on the field of pain 2,Monte Rosa's CDK2 molecular glue degraders 3,Eisbach's ALC1 inhibitor 4,Roche's tri-complex inhibitors 5,Eli Lilly’s CRHR2 peptide agonists 6,LP(a) inhibitor from CSPC is better?