Request Demo
Recent blog posts
Pharma Pioneer
8 min read
Stop Drowning in Data: Meet Synapse Lens, the Ultimate Reading Assistant for Biomed Professionals
5 February 2026
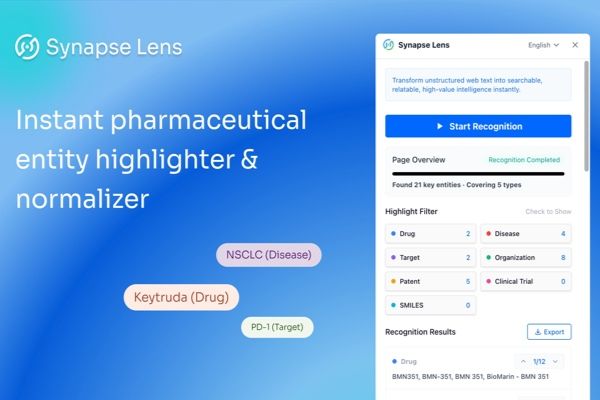
If you’ve ever felt overwhelmed by the sheer volume of reading required to stay ahead, there is a tool designed specifically for you. Introducing Synapse Lens, a Chrome extension hailed as a "superpower" for pharmaceutical and biotech professionals.
Pharma Pioneer
2 min read
Rise Therapeutics Begins Rheumatoid Arthritis Trial with First Patient
31 May 2024
Rise Therapeutics has made a significant step forward by enrolling the first participant in its Phase 1 clinical trial for R-2487, a new oral immunotherapy for rheumatoid arthritis (RA).
Pharma Pioneer
3 min read
Promising Early Results in NSCLC Trial with Deltacel-01 from Kiromic BioPharma
31 May 2024
Kiromic BioPharma, Inc. has announced promising initial findings from its Phase 1 clinical study of Deltacel™ in treating advanced non-small cell lung cancer (NSCLC).
Pharma Pioneer
2 min read
Eilean Therapeutics Launches Clinical Trial for Eiletoclax, a Safer BCL2 Inhibitor
31 May 2024
Eilean Therapeutics announced that it has received approval from the AHREC to commence human trials for eiletoclax, a selective and powerful BCL2 inhibitor, in its Phase 1 clinical program.
Pharma Pioneer
2 min read
Eilean Therapeutics Advances Lomonitinib Clinical Trials with Single and Multiple Dose Studies
31 May 2024
Eilean Therapeutics LLC has successfully concluded a Phase 1 clinical trial for a drug named lomonitinib and is now starting a multiple ascending dose Phase 1 trial.
Pharma Pioneer
2 min read
Positive Phase 1b Results for ARM210 in RYR1-Related Myopathies Treatment
31 May 2024
ARMGO Pharma has announced the successful results of a Phase 1b clinical trial for Rycal® ARM210, a treatment aimed at Ryanodine Receptor 1 Related Myopathies (RYR1-RM).
Pharma Pioneer
4 min read
FDA Approves Gracell's Phase 1 Trial for Early-Line Multiple Myeloma FasTCAR-T Therapy
31 May 2024
FDA has cleared Gracell’s Investigational New Drug (IND) application, allowing the Company to initiate a Phase 1 clinical trial of GC012F in the United States for the early-line treatment of multiple myeloma (ELMM).
Pharma Pioneer
3 min read
Navigating the Future of Decentralized Clinical Trials
31 May 2024
The COVID-19 pandemic has significantly transformed the healthcare industry, particularly in the realm of pharmaceutical clinical trials.
Pharma Pioneer
3 min read
EicOsis Launches EC5026 Phase 1b Trial
31 May 2024
EC5026, characterized by its potency and selectivity as an sEH inhibitor, is pivotal in managing the metabolism of signaling lipids and addressing inflammation and stress responses triggered by injury or illness.
Pharma Pioneer
2 min read
Promising Early Results for Ziftomenib Combo in Treating AML
31 May 2024
Kura Oncology, Inc. announced promising preliminary results from the KOMET-007 Phase 1 dose-escalation trial.
Pharma Pioneer
3 min read
Uvax Bio Initiates Phase 1 Trial for HIV-1 Vaccine Candidates
31 May 2024
Uvax Bio, LLC has commenced a Phase 1 clinical trial in Australia for its HIV-1 vaccine candidates, UVAX-1107 and UVAX-1197.
Pharma Pioneer
3 min read
Lexaria Files IND Application for DehydraTECH-CBD Hypertension Trial
31 May 2024
Lexaria Bioscience Corp. submitted its Investigational New Drug (IND) application to the U.S. Food and Drug Administration (FDA) for its upcoming clinical trial, HYPER-H23-1.