Advancements in AML and MDS Treatment: The Rapid and Efficient UltraCAR-T Therapy with PRGN-3006
The UltraCAR-T platform offers a solution by simplifying the manufacturing process, allowing local production in a hospital's GMP facility immediately after apheresis. This platform uses Precigen's cutting-edge non-viral gene delivery system and a rapid manufacturing method that ensures high cell viability, enabling the administration of autologous CAR-T cells just one day post gene transfer.
PRGN-3006 UltraCAR-T cells, developed with Precigen's UltraVector platform, feature the co-expression of CD33 CAR, membrane-bound IL-15 (mbIL15), and a kill switch. The inclusion of mbIL15 provides the cells with an internal cytokine source, enhancing their expansion and persistence without the need for additional cytokines. This also negates the need for ex vivo T cell expansion before administration. The kill switch co-expression offers a mechanism for the controlled elimination of PRGN-3006 post-treatment, enhancing therapeutic safety.
Manufactured using a non-viral method and a streamlined process, PRGN-3006 UltraCAR-T cells were confirmed to express CAR, mbIL15, and the kill switch through flow cytometry and western blot analysis. In vitro tests showed robust expansion in the presence of the CD33 antigen, no autonomous expansion without it, and sustained persistence without exogenous cytokines. The cells demonstrated specific killing of CD33+ tumor cells and released inflammatory cytokines when co-cultured with AML tumor cells. The kill switch activator antibody treatment effectively eliminated PRGN-3006 cells.
In an aggressive AML xenograft model using immunocompromised mice, a single administration of PRGN-3006 UltraCAR-T cells one day after gene transfer significantly reduced tumor burden and improved survival rates compared to conventional CAR-T cells lacking mbIL15 expression. The cells also showed higher engraftment, expansion, and persistence in the mice.
The FDA has granted approval for an investigational new drug application for PRGN-3006 UltraCAR-T, and a Phase 1 clinical trial for treating relapsed/refractory AML and high-risk myeloid dysplastic syndrome patients is underway (ClinicalTrials.gov Identifier: NCT03927261).
Disclosures indicate that several individuals are employed by Precigen, Inc., and one has equity ownership in Intrexon Corp., while another has a board membership with Celyad.
How to Use Synapse Database to Search and Analyze Translational Medicine Data?
The transational medicine section of the Synapse database supports searches based on fields such as drug, target, and indication, covering the T0-T3 stages of translation. Additionally, it offers a historical conference search function as well as filtering options, view modes, translation services, and highlights summaries, providing you with a unique search experience.
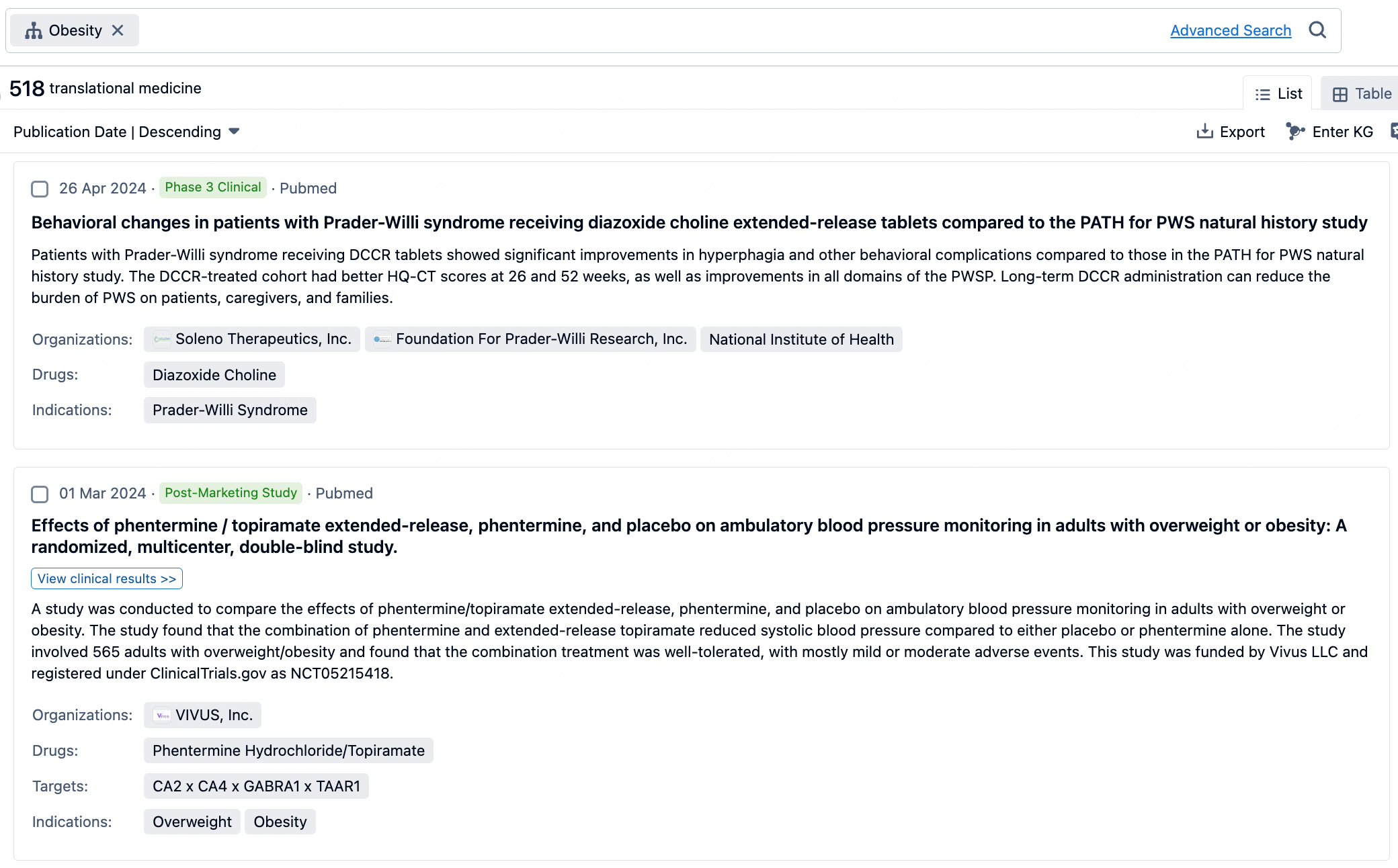
Taking obesity as an example, select "obesity" under the indication category and click search to enter the Translational Medicine results list page. By clicking on the title, you can directly navigate to the original page.
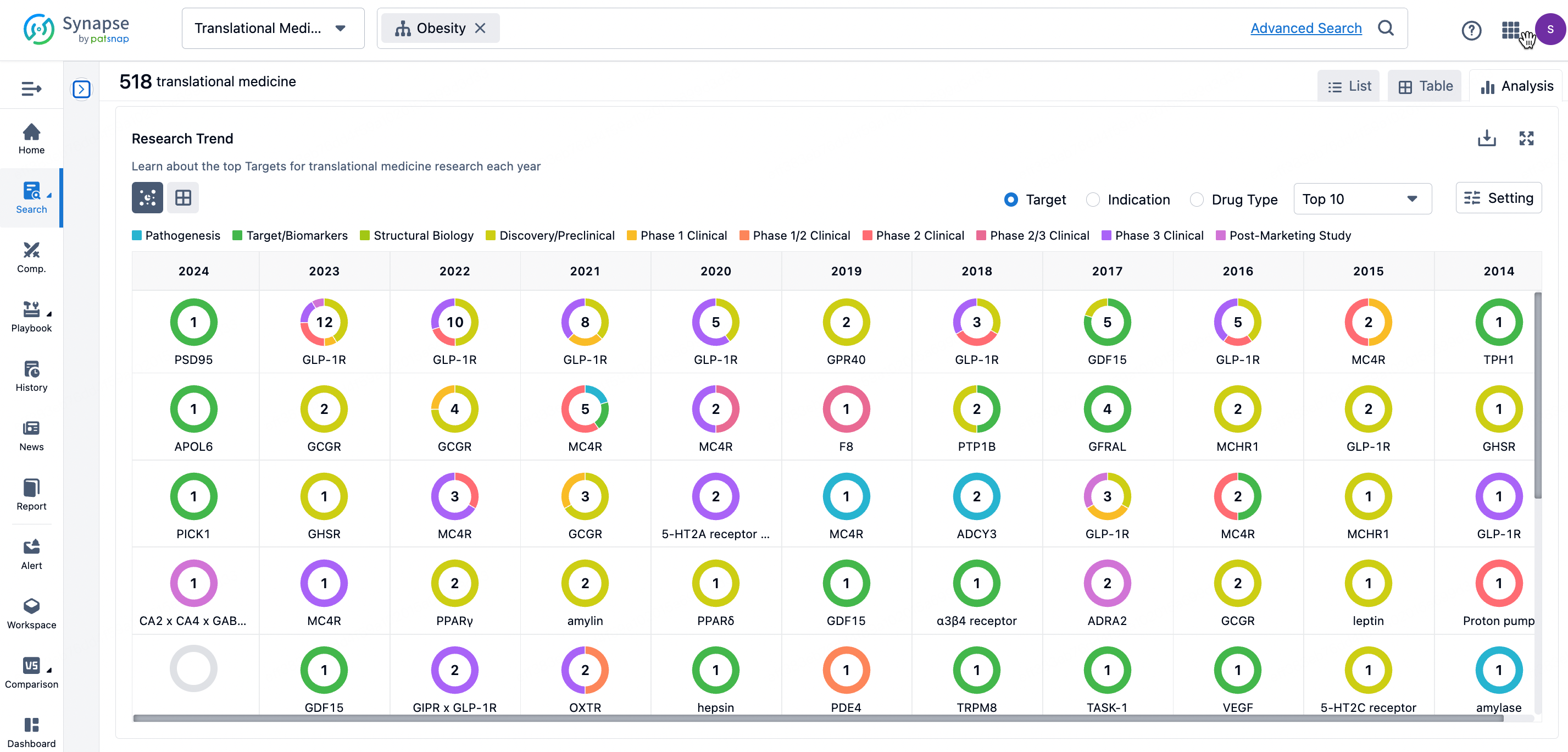
By clicking the analysis button, you can observe that GLP-1R treatment for obesity has gained significant attention over the past three years, with preclinical research still ongoing in 2023. Additionally, there are emerging potential targets, such as GDF15, among others.
Click on the image below to go directly to the Translational Medicine search interface.