Advancements in CDK4/6 Inhibition: The Emergence of FCN-437 for Solid Tumor Therapy
FCN-437 is presented as a selective and orally bioavailable CDK4/6 inhibitor with broad-spectrum anti-proliferating effects on Rb+ tumor cells from various solid tumors. It shows superior potency to ribociclib and comparable efficacy to palbociclib. In tumor xenograft models, FCN-437 significantly inhibited tumor growth, either matching or surpassing the approved inhibitors. The compound also synergizes well with letrozole or fulvestrant and exhibits favorable pharmacokinetics, including improved bioavailability and preferential distribution to brain tissues in rats, suggesting enhanced blood-brain barrier permeability.
Non-clinical studies indicate that FCN-437 has a favorable safety profile, with low hERG activity, no cardiotoxicity, and no CYP450 induction or inhibition, reducing the risk of drug interactions. Overall, FCN-437 is highlighted for its drug-like properties, enhanced efficacy, improved pharmacokinetics with BBB penetration, and preferable safety profiles. It is considered a promising candidate for targeted therapy for advanced solid tumors with brain metastases, either as a single agent or in combination. A phase 1 clinical trial for FCN-437 was approved by the NMPA in 2018.
How to Use Synapse Database to Search and Analyze Translational Medicine Data?
The transational medicine section of the Synapse database supports searches based on fields such as drug, target, and indication, covering the T0-T3 stages of translation. Additionally, it offers a historical conference search function as well as filtering options, view modes, translation services, and highlights summaries, providing you with a unique search experience.
Taking obesity as an example, select "obesity" under the indication category and click search to enter the Translational Medicine results list page. By clicking on the title, you can directly navigate to the original page.
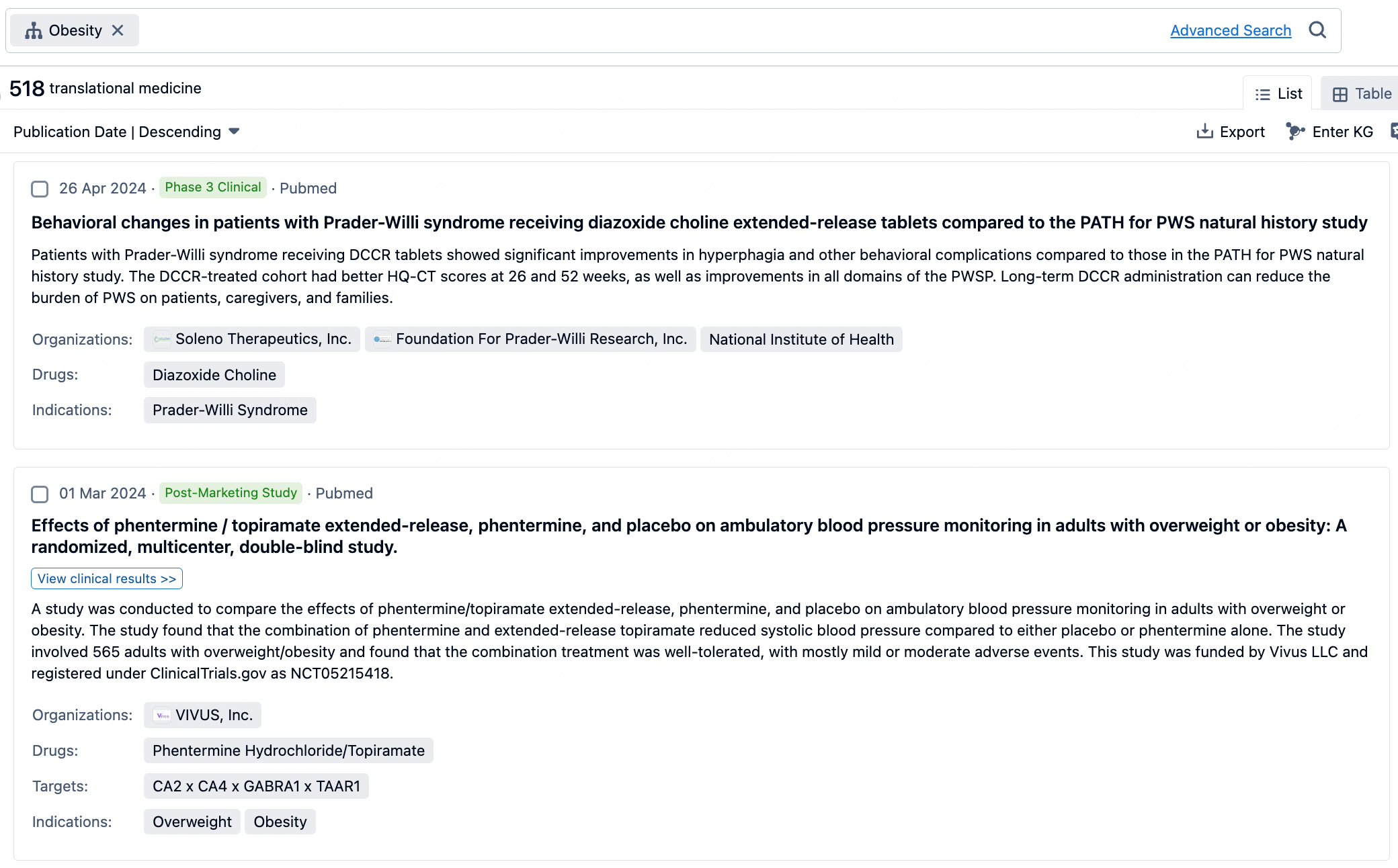
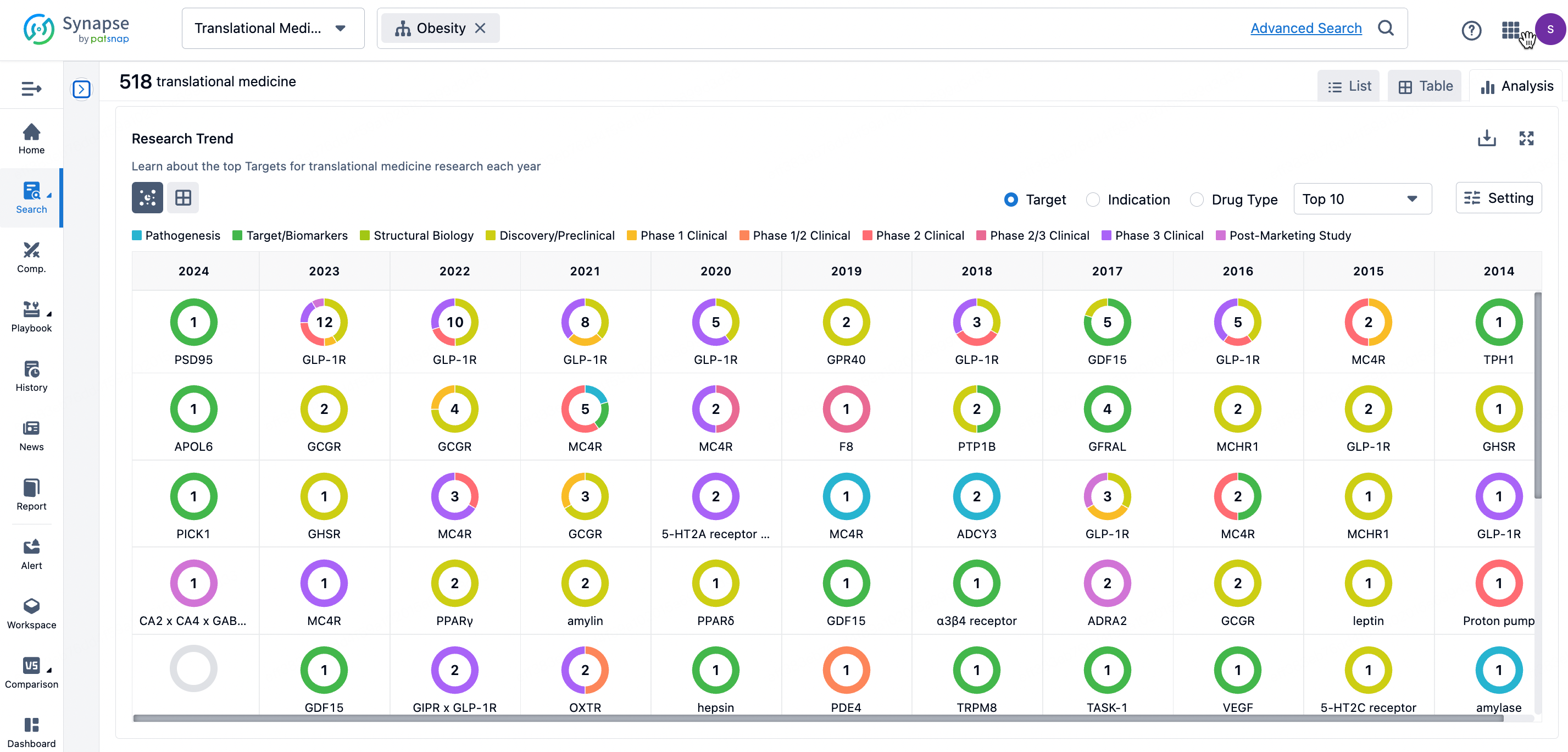
By clicking the analysis button, you can observe that GLP-1R treatment for obesity has gained significant attention over the past three years, with preclinical research still ongoing in 2023. Additionally, there are emerging potential targets, such as GDF15, among others.
Click on the image below to go directly to the Translational Medicine search interface.