ARQ 531: A Potent BTK Inhibitor Overcoming Ibrutinib Resistance in Chronic Lymphocytic Leukemia Mouse Model
In the preclinical phase, the potency and binding kinetics of ARQ 531 were evaluated through enzymatic and Surface Plasmon Resonance (SPR) assays. CLL B cells were treated with ARQ 531, and the impact on BCR signaling was assessed using immunoblotting. The migration of CLL cells towards CXCL12 and CXCL13 was measured by flow cytometry, and the viability of CLL cells was determined using Annexin V and propidium iodide staining.
In vivo studies were conducted using a TCL1 mouse model, where mice were treated with either ARQ 531 or ibrutinib after the establishment of a CD5+/CD19+ population in peripheral blood. The results indicated that ARQ 531 significantly reduced the viability of CLL cells at various concentrations and demonstrated a dose-dependent inhibition of BTK, AKT, and ERK phosphorylation.
Furthermore, ARQ 531 was found to decrease the expression of activation markers induced by CpG ligand and reduce the migration of CLL cells in response to chemokines. Notably, ARQ 531 was effective against both wild type and C481S mutated BTK, with an IC50 of less than 1nM in biochemical assays and a residence time of 51 minutes for wild type BTK and 56 minutes for the mutated variant.
In the TCL1 mouse model, ARQ 531 showed superior survival outcomes compared to ibrutinib and the control group, with a median survival that had not been reached by 74 days at the higher doses of ARQ 531. The treatment also led to lower lymphocyte counts and reduced CD5+/CD19+ populations, along with an increase in absolute neutrophil count over time.
Pharmacokinetic data from a single oral dose study in monkeys showed a bioavailability of 72.4%, a maximum concentration (Cmax) of 9μM, and a half-life exceeding 24 hours.
In conclusion, ARQ 531 exhibits promising in vitro and in vivo activity as a BTK inhibitor, with the ability to inhibit multiple pathways involved in CLL cell activation, migration, and viability. Its effectiveness against C481S mutated BTK variants and its superior efficacy in the TCL1 mouse model suggest that ARQ 531 may be a valuable therapeutic option for CLL patients, including those resistant to ibrutinib. The findings support further preclinical development and eventual transition to clinical trials.
How to Use Synapse Database to Search and Analyze Translational Medicine Data?
The transational medicine section of the Synapse database supports searches based on fields such as drug, target, and indication, covering the T0-T3 stages of translation. Additionally, it offers a historical conference search function as well as filtering options, view modes, translation services, and highlights summaries, providing you with a unique search experience.
Taking obesity as an example, select "obesity" under the indication category and click search to enter the Translational Medicine results list page. By clicking on the title, you can directly navigate to the original page.
By clicking the analysis button, you can observe that GLP-1R treatment for obesity has gained significant attention over the past three years, with preclinical research still ongoing in 2023. Additionally, there are emerging potential targets, such as GDF15, among others.
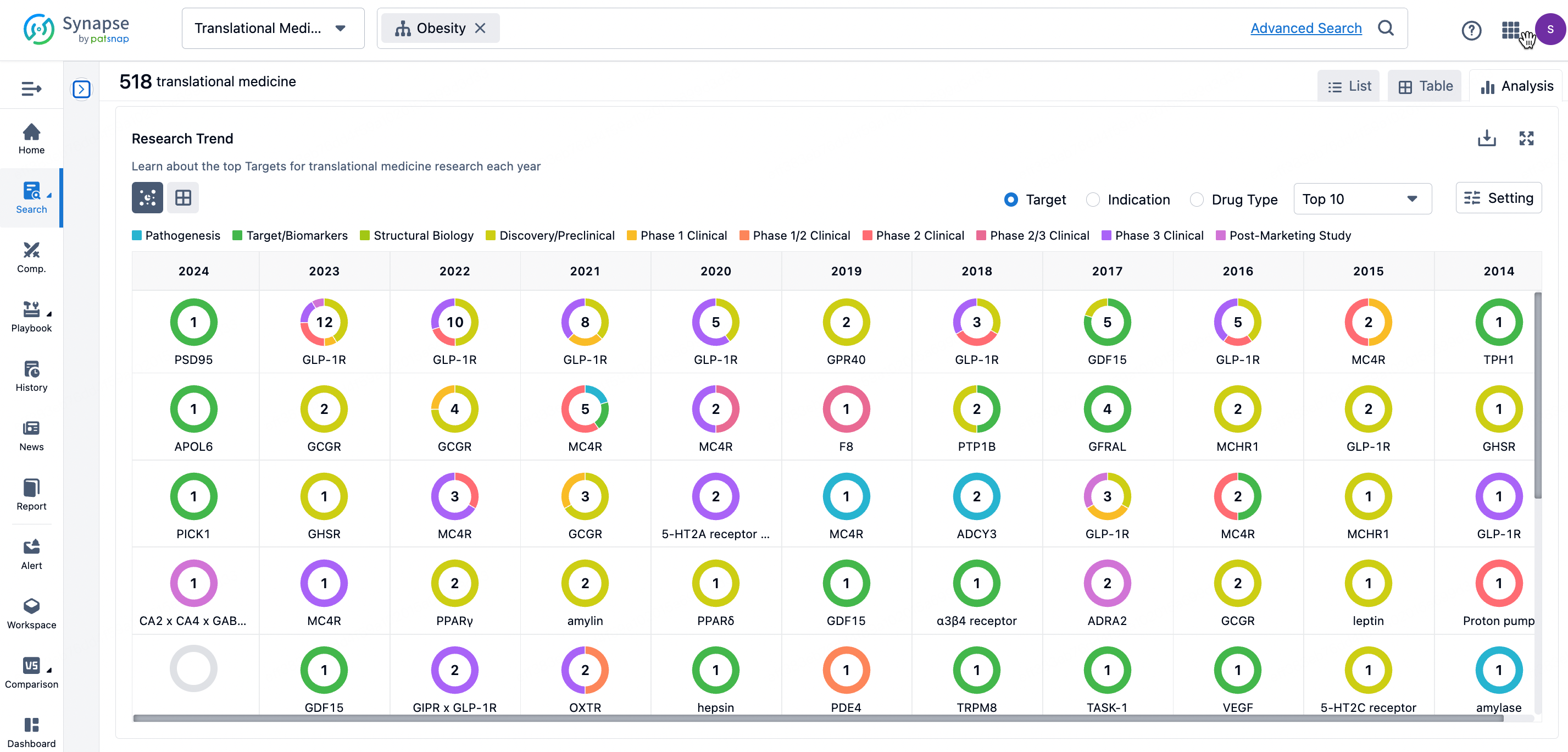
Click on the image below to go directly to the Translational Medicine search interface.