Harnessing PHE885: Advancing CAR-T Therapy for Multiple Myeloma with a Rapid and Potent BCMA Targeting Approach
The PHE885 CAR-T product is engineered with an extracellular scFv linked to the CD8α hinge and transmembrane domains, and includes CD137 (4-1BB) co-stimulatory and CD3ζ signaling domains. This construct was found to be superior in functional assays, demonstrating enhanced potency and the ability to maintain the stemness of T cells, which is crucial for a longer therapeutic response.
A key innovation in the development of PHE885 is the T-Charge™ manufacturing platform, which allows for a rapid production process of less than two days, avoiding the need for T cell expansion. This process results in CAR-T cells that retain a higher proportion of naive/stem cell memory T cells (Tscm), which are known for their engraftment and expansion capabilities.
In vitro and in vivo studies have shown that PHE885 secretes significantly more target-specific IL-2 and IFN-gamma compared to traditional manufacturing products. Moreover, in a mouse model of MM, PHE885 induced tumor regression at lower doses and demonstrated greater efficacy and cellular expansion than traditional products.
The enhanced potency and persistence of PHE885, attributed to its novel CAR construct and the T-Charge™ manufacturing process, have led to the initiation of a Phase 1 clinical trial (NCT04318327) for patients with relapsed/refractory MM. Initial findings from the trial's dose escalation phase will be presented, showcasing the potential of this innovative CAR-T therapy in treating MM.
How to Use Synapse Database to Search and Analyze Translational Medicine Data?
The transational medicine section of the Synapse database supports searches based on fields such as drug, target, and indication, covering the T0-T3 stages of translation. Additionally, it offers a historical conference search function as well as filtering options, view modes, translation services, and highlights summaries, providing you with a unique search experience.
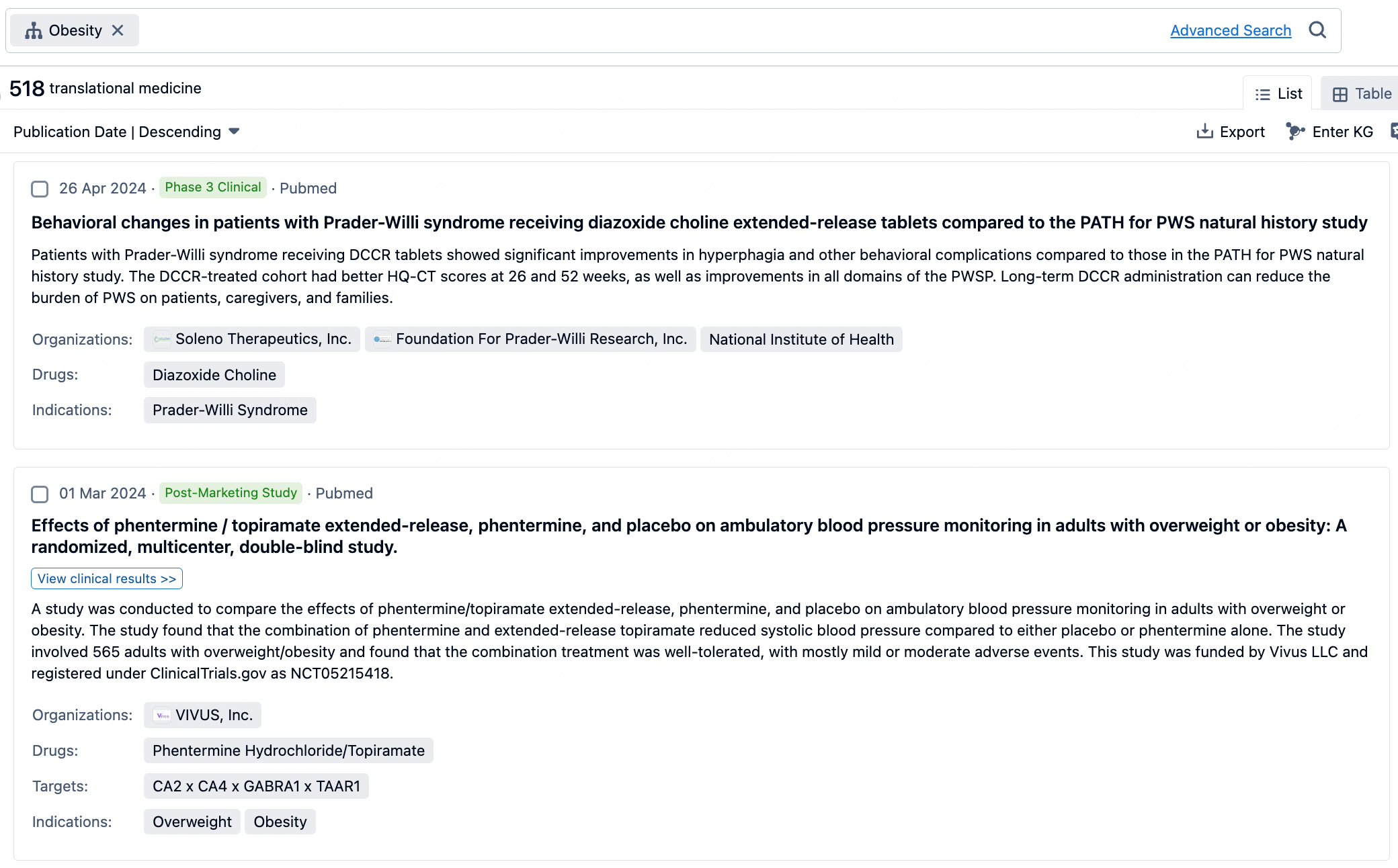
Taking obesity as an example, select "obesity" under the indication category and click search to enter the Translational Medicine results list page. By clicking on the title, you can directly navigate to the original page.
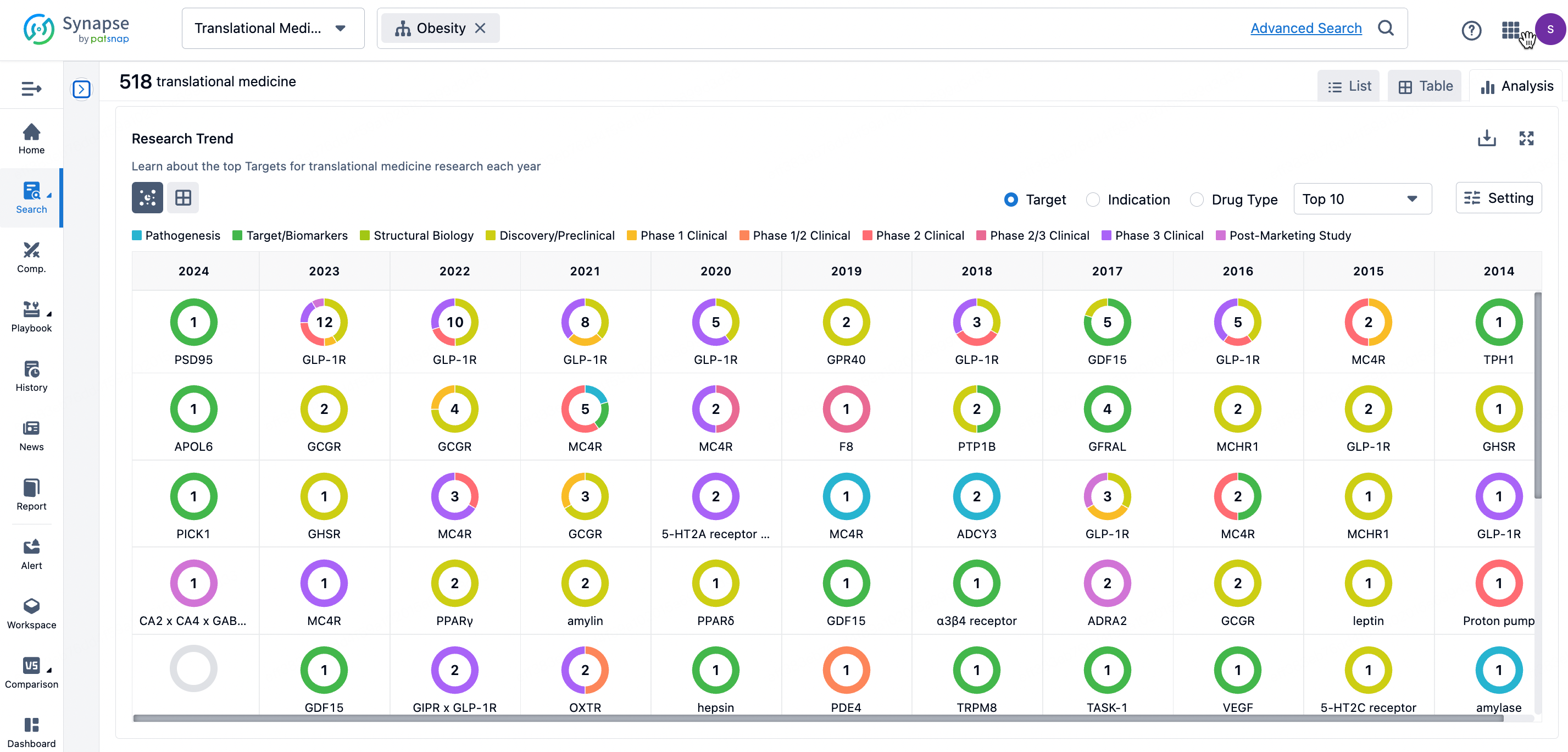
By clicking the analysis button, you can observe that GLP-1R treatment for obesity has gained significant attention over the past three years, with preclinical research still ongoing in 2023. Additionally, there are emerging potential targets, such as GDF15, among others.
Click on the image below to go directly to the Translational Medicine search interface.