Selective Regulatory T Cell Expansion with AMG 592: A Promising IL-2 Mutein for Inflammatory Diseases
In vitro, AMG 592 demonstrated a more selective activation of Tregs and reduced inflammatory cytokine production compared to standard IL-2. In animal models, it induced Treg expansion without significant changes in body temperature or C-reactive protein levels, unlike standard IL-2.
The phase 1 clinical trial involved healthy volunteers receiving various doses of AMG 592 or a placebo. The compound was found to be well tolerated, with the most common side effect being mild, localized skin reactions. Pharmacokinetic data showed an increase in serum levels of AMG 592 that correlated with the administered dose. It also led to a significant and sustained increase in the ratio of Tregs to conventional T cells, with the peak effect observed around day 8 post-injection.
Importantly, AMG 592 did not alter NK cell numbers or significantly increase conventional T cell counts. Furthermore, it did not elevate levels of pro-inflammatory cytokines, suggesting a safer therapeutic profile than conventional IL-2.
The conclusion of the study is that AMG 592 selectively expands Tregs in a dose-dependent manner without causing a systemic inflammatory response. This suggests that AMG 592 may offer a broader therapeutic window and potentially allow for less frequent dosing. The study advocates for further research into the use of AMG 592 in treating inflammatory and autoimmune diseases by leveraging the immune-regulating properties of Tregs.
How to Use Synapse Database to Search and Analyze Translational Medicine Data?
The transational medicine section of the Synapse database supports searches based on fields such as drug, target, and indication, covering the T0-T3 stages of translation. Additionally, it offers a historical conference search function as well as filtering options, view modes, translation services, and highlights summaries, providing you with a unique search experience.
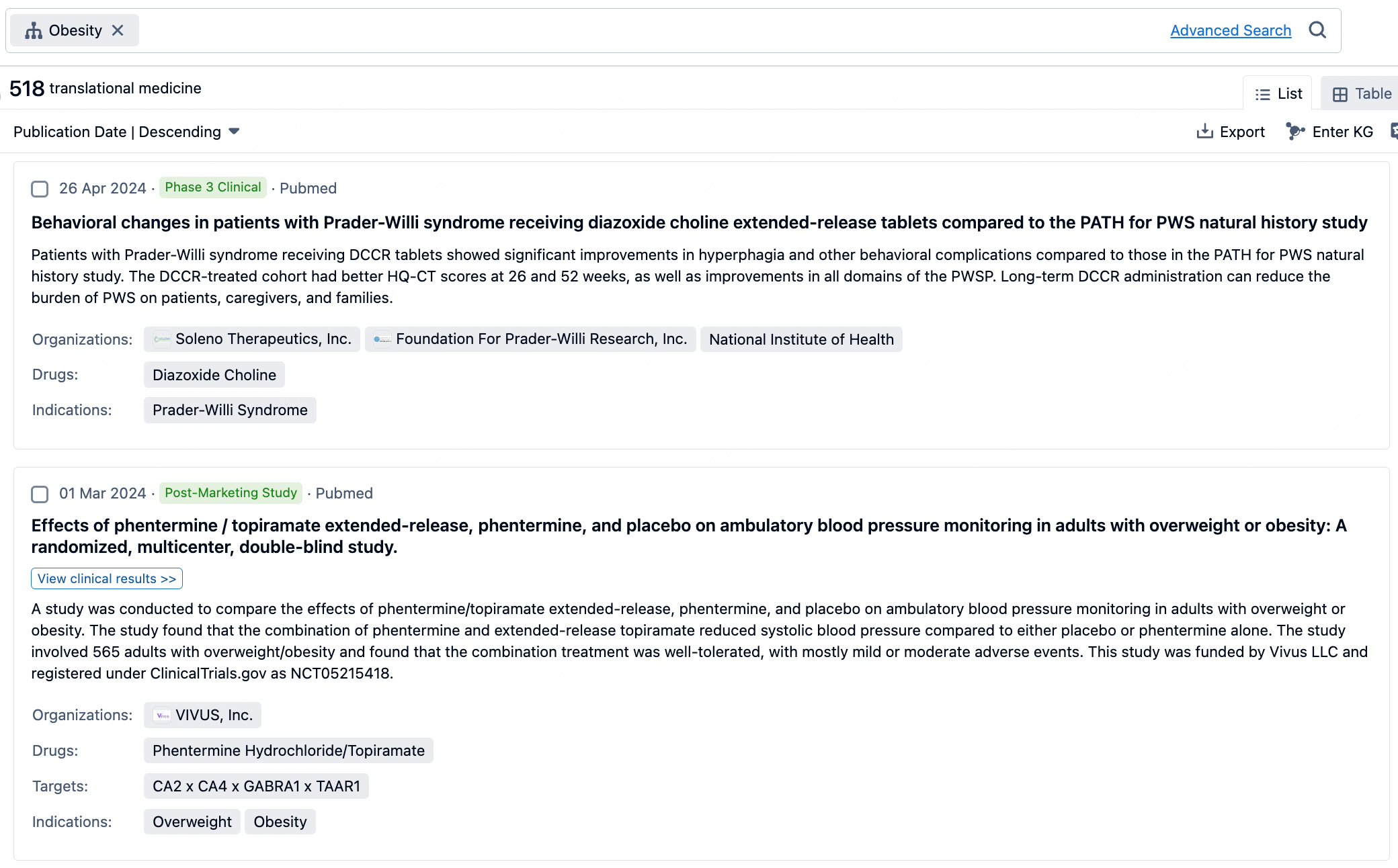
Taking obesity as an example, select "obesity" under the indication category and click search to enter the Translational Medicine results list page. By clicking on the title, you can directly navigate to the original page.
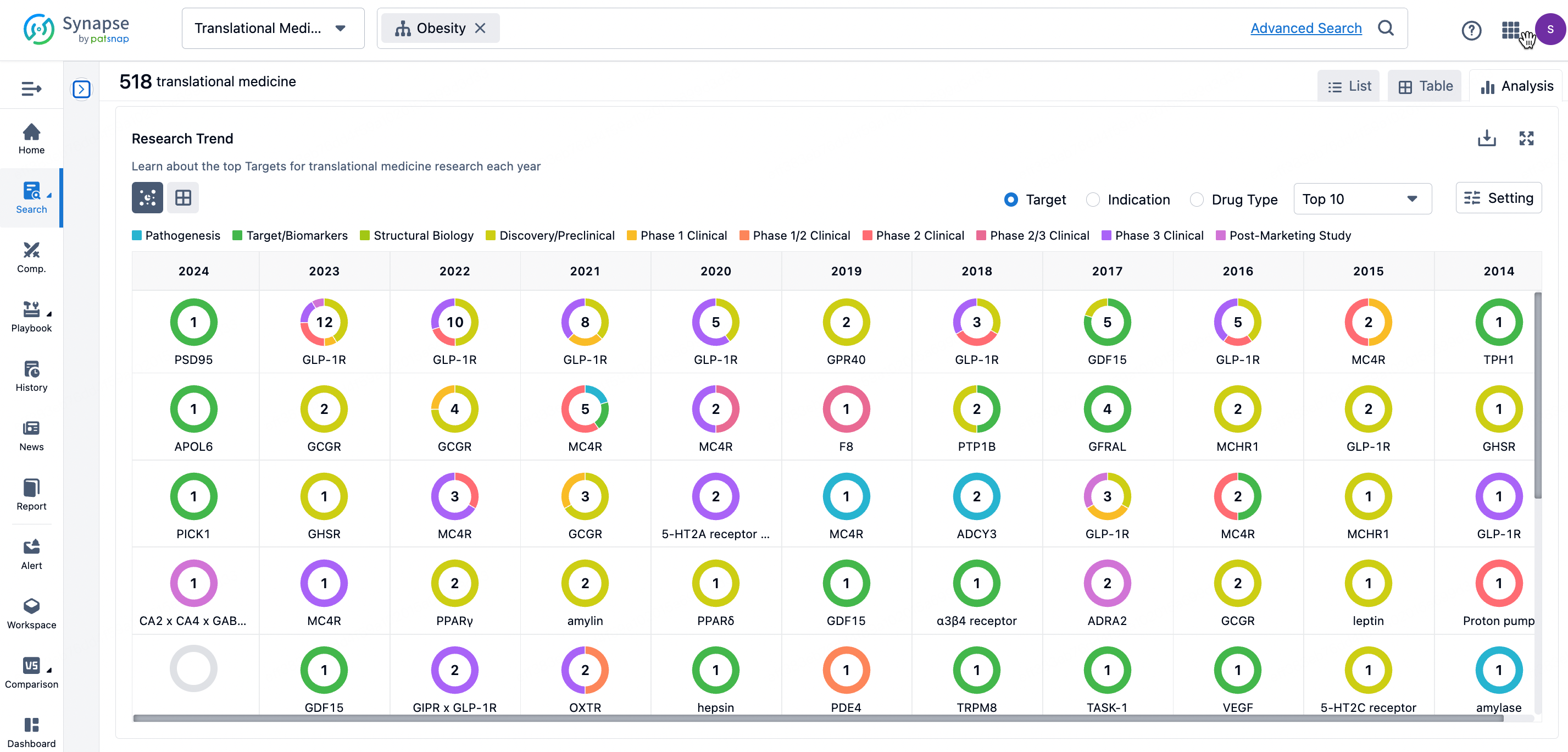
By clicking the analysis button, you can observe that GLP-1R treatment for obesity has gained significant attention over the past three years, with preclinical research still ongoing in 2023. Additionally, there are emerging potential targets, such as GDF15, among others.
Click on the image below to go directly to the Translational Medicine search interface.