10xBio's Phase 2b Trial Suggests Improved Submental Fat Treatment
10xBio, LLC, a firm specializing in biotechnology, has unveiled early clinical trial data pertaining to its groundbreaking injection-based treatment, known as 10XB101, intended for the reshaping of the submental region. As a cutting-edge substitute for existing fat reduction methods, 10XB101 demonstrates heightened effectiveness and minimal adverse reactions, while also improving the overall experience for patients and offering a quicker course of therapy.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
In this methodically structured, randomized, and placebo-controlled trial with three active treatment arms, participants were administered multiple doses of 10XB101 following a strategically determined sequence, undergoing as many as six sessions with an interval of four weeks between each. The effectiveness of the treatment was measured through clinical and self-report scales specifically targeting submental adiposity, benchmarking the initial state against the condition four weeks post-final treatment.
"The outcomes from this trial unequivocally indicate a higher level of effectiveness and better tolerance for 10XB101 in comparison to deoxycholate, a compound I have extensive experience with, exceeding over a decade," commented Mitchel P. Goldman, M.D., an expert dermatologic surgeon and the medical director at Cosmetic Laser Dermatology.
"10XB101 is ideally suited for the substantial market potential in submental sculpting. Further, its characteristics show potential for broader applications in body sculpting procedures, including treatments targeting the abdominal area and the flanks," elaborated Dr. Goldman.
The findings are significantly more favorable when compared to deoxycholate, which is the singular drug approved for non-surgical adiposity reduction in the United States, presenting up to a fourfold increase in patients who saw at least a two-grade improvement as per the Submental Fat Rating Scale/Patient Submental Fat Rating Scale. Treatment with 10XB101 resulted in substantially fewer and less severe reactions at injection sites than its market counterpart.
On March 9, at the American Academy of Dermatology's Annual Meeting in 2024, Kavita Darji, MD, FAAD, a cosmetic dermatologic surgeon with a fellowship background, showcased these research findings and provided a platform for discussion and in-depth queries post-presentation.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
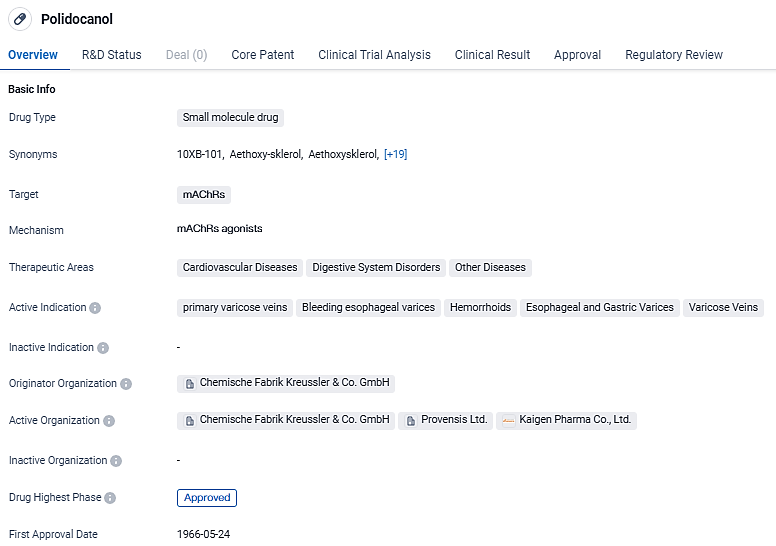
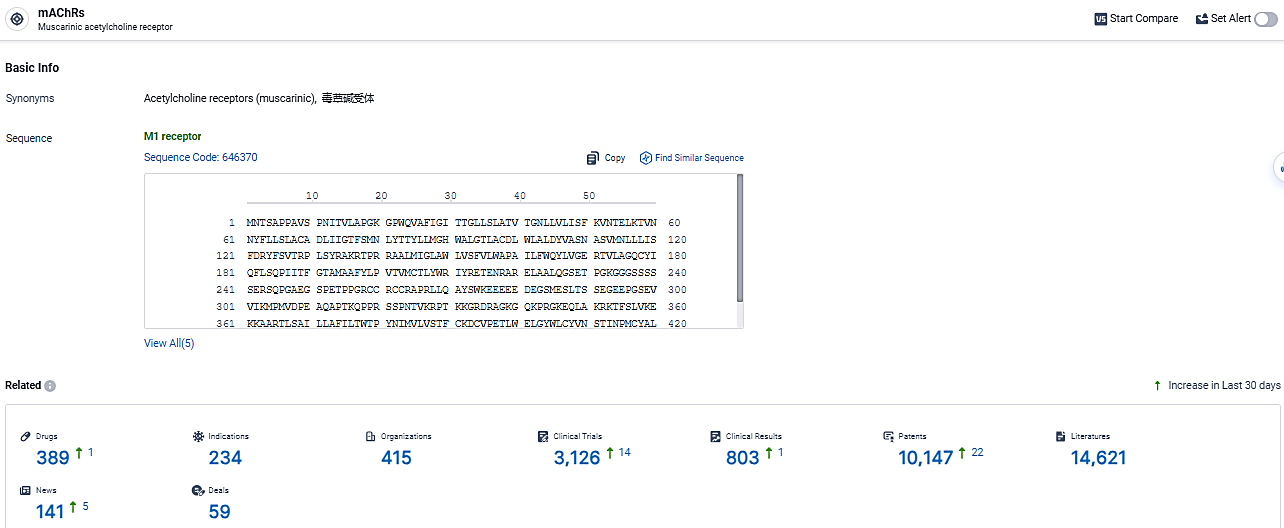
According to the data provided by the Synapse Database, As of March 13, 2024, there are 389 investigational drugs for the mAChRs target, including 234 indications, 415 R&D institutions involved, with related clinical trials reaching 3126, and as many as 14621 patents.
Polidocanol is a small molecule drug that targets mAChRs and is used in the treatment of various cardiovascular diseases, digestive system disorders, and other diseases. Polidocanol is primarily used for the treatment of varicose veins, bleeding esophageal varices, hemorrhoids, esophageal and gastric varices, and other related conditions. As an orphan drug, it is subject to specific regulations aimed at supporting the development and availability of medications for rare diseases.