2023 AAO | Innovent Biologics Announces Latest Clinical Data for Dual-Target Ophthalmic New Drugs IBI302 and IBI324
On November 6, 2023, Innovent Biologics announced two latest clinical research results at the 2023 American Academy of Ophthalmology (AAO) annual meeting. They are the Phase 2 clinical data of anti-VEGF-anti-complement dual target drug (IBI302) for the treatment of neovascular age-related macular degeneration (nAMD), and the Phase 1 clinical data of anti-VEGF-A/Ang-2 bispecific antibody (IBI324) for the treatment of diabetic macular edema (DME).
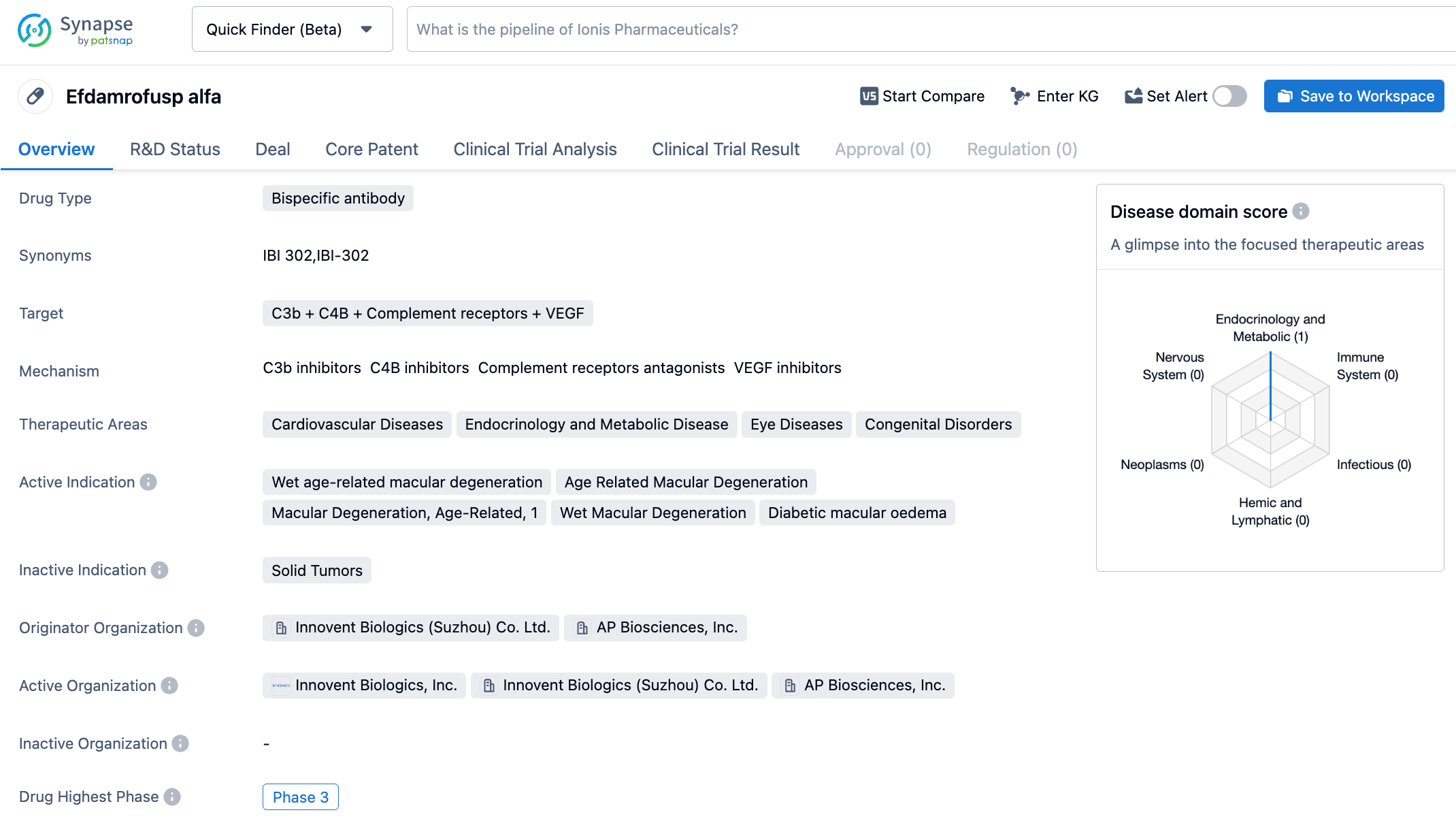
IBI302 is a bispecific recombinant human fusion protein with global intellectual property rights owned by Innovent Biologics. It targets C3b + C4B + Complement receptors + VEGF. The N-terminal is the VEGF binding domain, which can bind to the VEGF family to block VEGF-mediated signaling pathways, inhibit the survival and proliferation of vascular endothelial cells, thereby inhibiting angiogenesis, reducing vascular permeability, and reducing vascular leakage. The C-terminal is the complement binding domain, which can inhibit the activation of the classical pathway and the bypass of the complement by specifically binding to C3b and C4b, reducing the inflammation reaction mediated by complement activation. IBI302 may exert a therapeutic effect by simultaneously inhibiting VEGF-mediated angiogenesis and complement activation pathways.
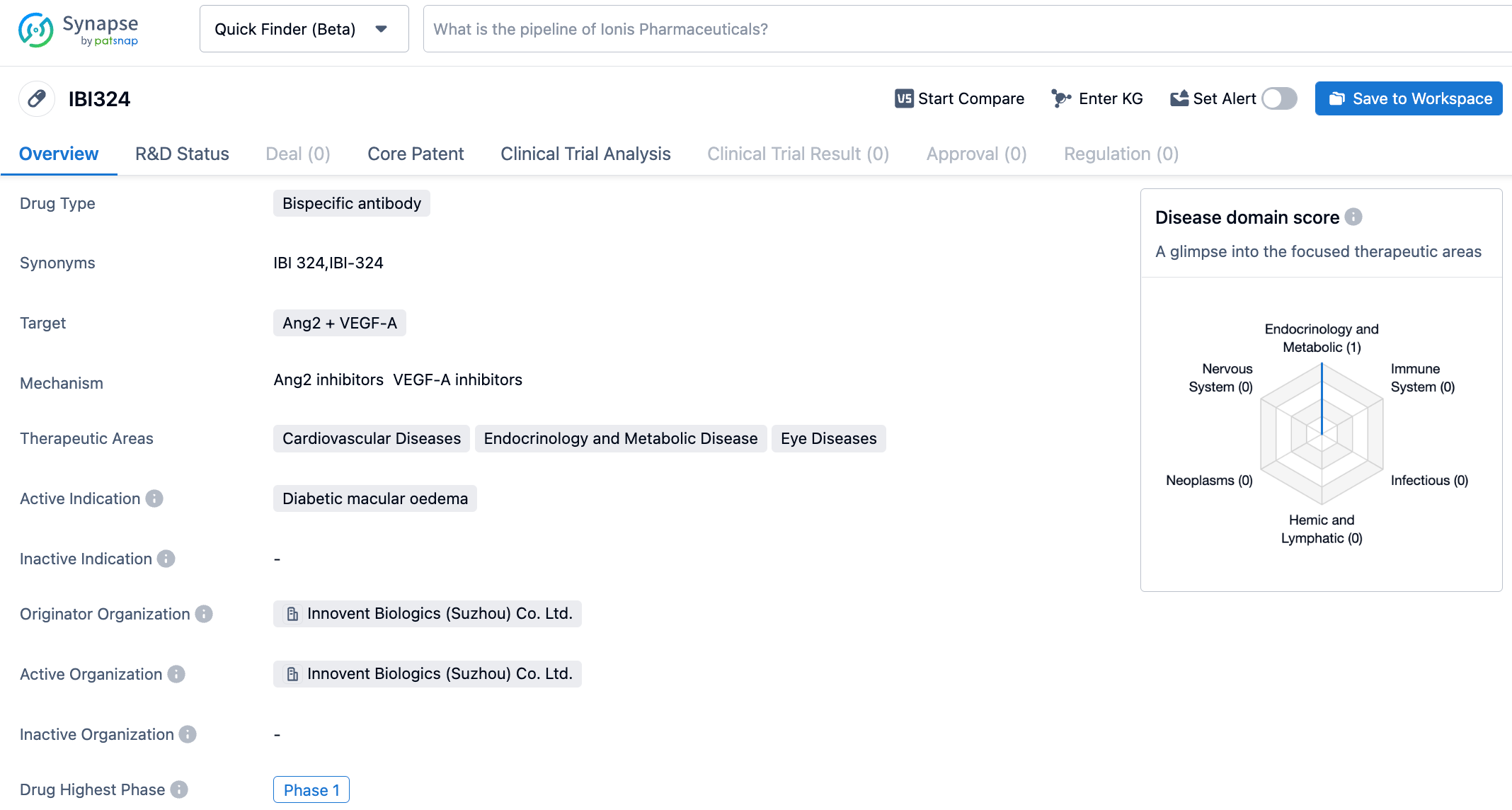
IBI324 is a recombinant fully humanized bispecific antibody targeting Ang2 + VEGF-A independently developed by Innovent Biologics. The N-terminal can block VEGF-A-mediated signaling pathways, inhibit the survival and proliferation of vascular endothelial cells, thereby inhibiting angiogenesis and reducing angiogenic leakage. The C-terminal is an anti-Ang-2 binding domain, which is an anti-Ang-2 selected by Innovent and does not bind to Ang-1. It can inhibit the binding of Ang-2 to the Tie-2 receptor, improve sensitivity to other inflammatory factors, further stabilize blood vessels, and inhibit vascular leakage. IBI324 may provide more clinical benefits for patients with DME by simultaneously blocking VEGF-A and Ang-2.
The Phase 2 latest results of IBI302 treatment for neovascular age-related macular degeneration (nAMD) were announced at the conference. The trial aimed to evaluate the effectiveness and safety of IBI302 in treating nAMD. 231 nAMD subjects were randomized 1:1:1 to receive 2mg IBI302, 4mg IBI302, or 2mg Aflibercept (three injections once every four weeks, followed by dosing every eight weeks, lasting for 52 weeks). The primary end result of the study was the change in best corrected visual acuity (BCVA) of the study eye at the 36th week. The results demonstrated that IBI302 2mg/4mg was non-inferior to Aflibercept 2mg in improving BCVA at week 36. By week 36, the mean changes in BCVA from baseline for IBI302 2mg, IBI302 4mg, and Aflibercept 2mg were +10.6, +11.4, and +12.0 letters, respectively. Improvements in Central Subfield Thickness (CST) measured by OCT were comparable across all groups. Preliminary signals were observed for preventing macular atrophy and fibrosis with IBI302. At week 52, the rates of macular atrophy on OCT for IBI302 2mg, IBI302 4mg, and Aflibercept 2mg were 5.2%, 5.2%, and 9.1%, respectively. The rates of color fundus fibrosis were 16.9%, 11.7%, and 14.3%, respectively. In terms of safety, IBI302 2mg, IBI302 4mg, and Aflibercept 2mg were found to be similar. No cases of endophthalmitis or retinal vasculitis occurred in the IBI302 groups.
The Phase 1 latest results of IBI324 treatment for diabetic macular edema (DME) were announced at the conference. The study aimed to evaluate the safety, tolerability, and effectiveness of IBI324 in treating DME. The study was divided into two phases: a single ascending dose (SAD) stage and a multiple ascending dose (MAD) stage. The results showed that the maximum tolerated dose was 4mg/eye. No serious ocular adverse events, endophthalmitis, or dose-limiting toxicities occurred during the study period. At day 42 post-dose in the SAD phase, BCVA and CST in IBI324 dosing groups had some improvements from baseline. Following the third dose at week 4 (day 84) in the MAD phase, BCVA improvements from baseline in IBI324 2mg and 4mg were 6.7±5.4 and 7.7±4.7 letters, respectively.
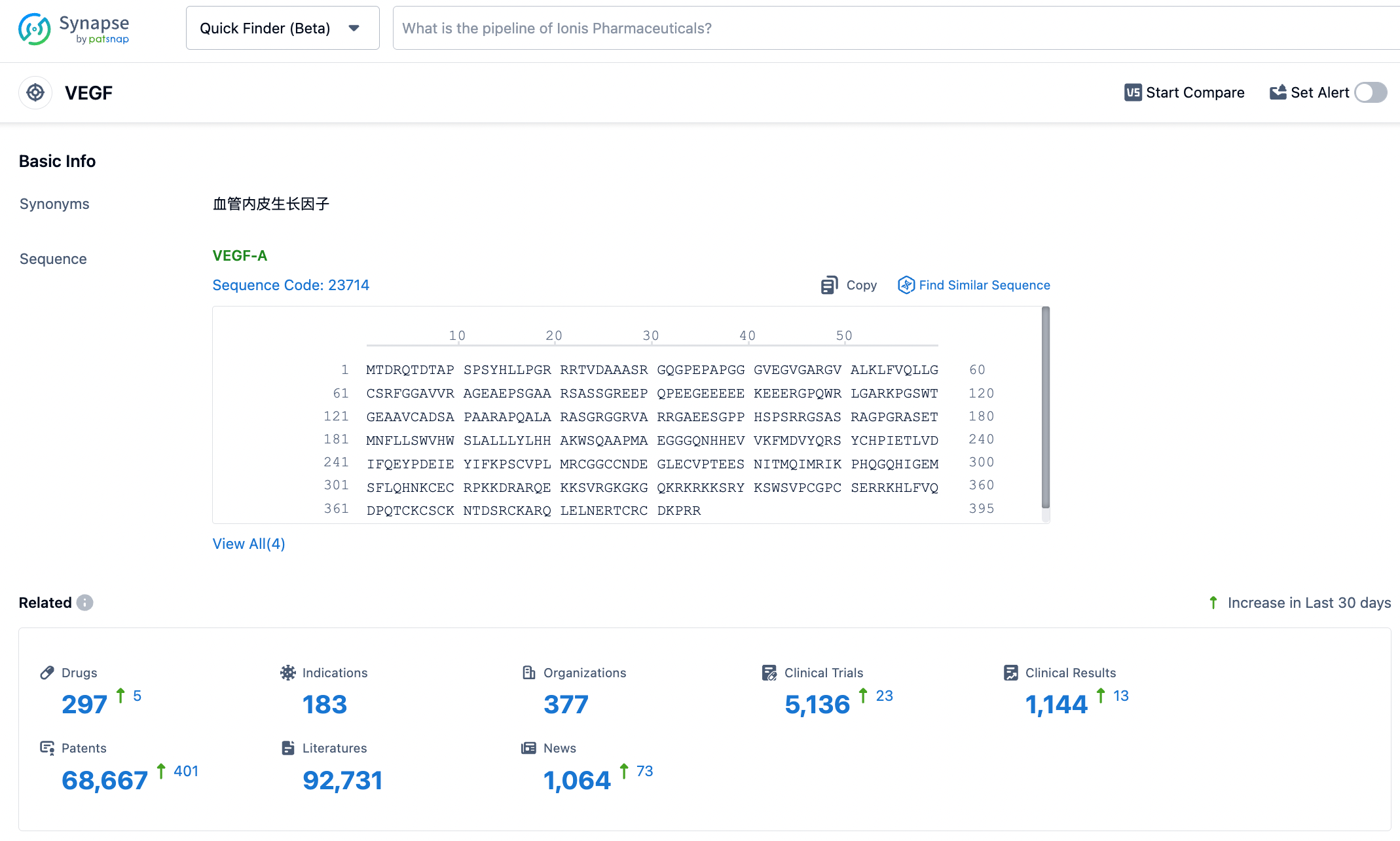
According to the Synapse database, as of November 7, 2023, there are 297 drugs under investigation for VEGF targets, applicable to 183 types of diseases, being developed by 377 institutions, involving 5133 relevant clinical trials with as many as 68,664 patents. The development of multi-target drugs based on anti-VEGF is currently the trend in the drug development for posterior ocular diseases. Innovent Biologics’ two ophthalmology products, IBI302 and IBI324, have each shown good efficacy in patients with nAMD and DME, with no new safety risk signals identified. We look forward to providing more choices for clinical treatment of a wide range of patients with posterior ocular diseases through the exploration of multi-target drugs.