2023 WCLC | AstraZeneca's EGFR-TKI Osimertinib Mesylate New Indication Phase 1b Clinical Data Selected for Oral Presentation
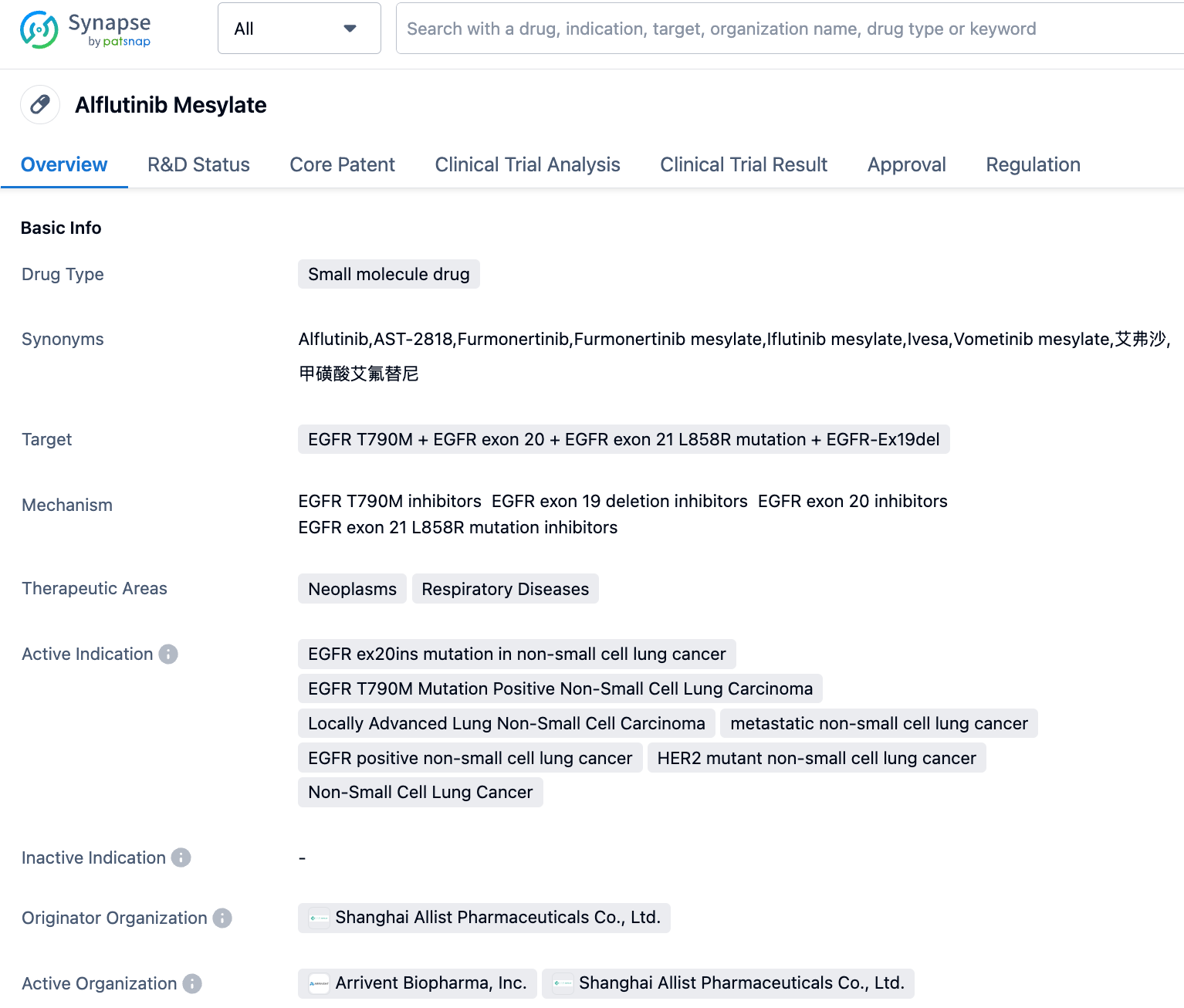
In September 2023, the interim analysis results of the FAVOUR study on Alflutinib Mesylate, developed by Shanghai Allist Pharmaceuticals and ArriVent Biopharma for the treatment of EGFR exon 20 insertion-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC), were presented orally at the 2023 World Conference on Lung Cancer (WCLC).
Alflutinib, a highly selective and irreversible third-generation epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), independently developed by Shanghai Allist Pharmaceuticals, is primarily used for the treatment of EGFR-mutated NSCLC.
Alflutinib was first approved in China in March 2021 for the treatment of adults with locally advanced or metastatic NSCLC who had disease progression during or after EGFR-TKI therapy and were confirmed to have an EGFR T790M positive mutation. In June 2022, the drug was granted approval by the NMPA for the first-line treatment of adults with locally advanced or metastatic NSCLC with an EGFR exon 19 deletion mutation (19DEL) or exon 21 substitution mutation (21L858R). In June 2021, ArriVent Biopharma entered into a licensing partnership with Allist, securing the exclusive rights to develop and commercialize Alflutinib in all territories outside of China (Mainland China, Hong Kong, Macau, and Taiwan).
The FAVOUR study presented at the conference is a multi-center, randomized, open-label Phase 1b clinical trial that enrolled locally advanced or metastatic NSCLC patients with EGFR exon 20 insertion mutations. Patients were randomly assigned to different dosage groups of Alflutinib Mesylate. As of June 15, 2023, a total of 86 participants were enrolled for safety assessment, and a total of 80 evaluable patients were available for efficacy analysis. The results from the Independent Radiological Review Committee (IRC) showed that the percent of patients who achieved a confirmed Objective Response Rate (ORR) in the initial treatment 240mg group, previously treated 240mg group, and previously treated 160mg group were 78.6%, 46.2%, and 38.5% respectively. The median Duration of Response (DoR) were 15.2 months, 13.1 months, and 9.7 months respectively.
Alflutinib Mesylate demonstrated antitumor activity across all EGFR exon 20 mutation subtypes in the near, far and helical regions. Alflutinib has shown good efficacy and predictable, manageable safety in patients with NSCLC harboring EGFR Exon20ins mutations. Based on these results, a global phase III trial (FURVENT/FURMO-004; NCT05607550) for locally advanced or metastatic NSCLC patients with EGFR Exon20ins mutations has been initiated.
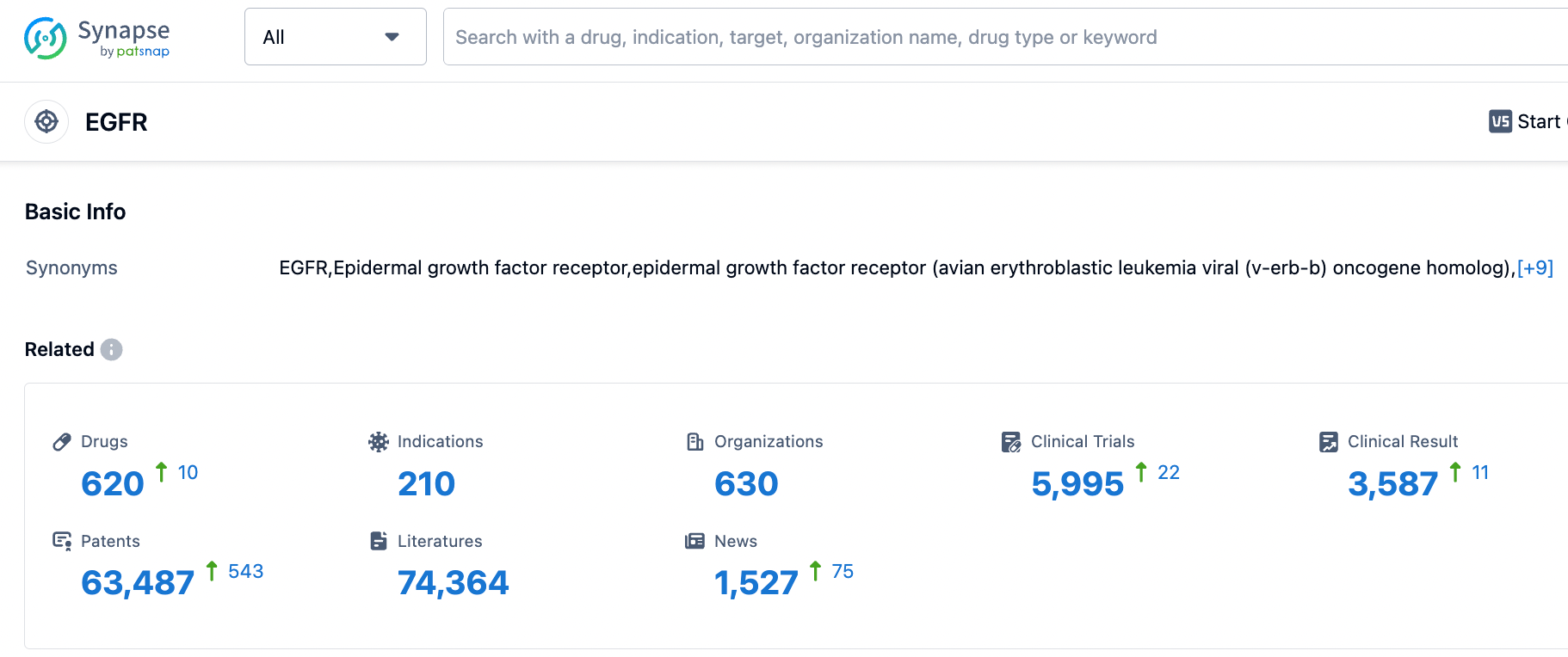
According to the information disclosed by the Synapse database, as of September 12, 2023, there are a total of 619 drugs under investigation targeting EGFR, covering 211 types of indications. There are 630 research institutions involved, with related clinical trials numbering 5992 and patents as many as 63464. We look forward to more EGFR drugs being approved for market access, providing more treatment options for patients.