A Comprehensive Review of Flurandrenolide's R&D Innovations
Flurandrenolide's R&D Progress
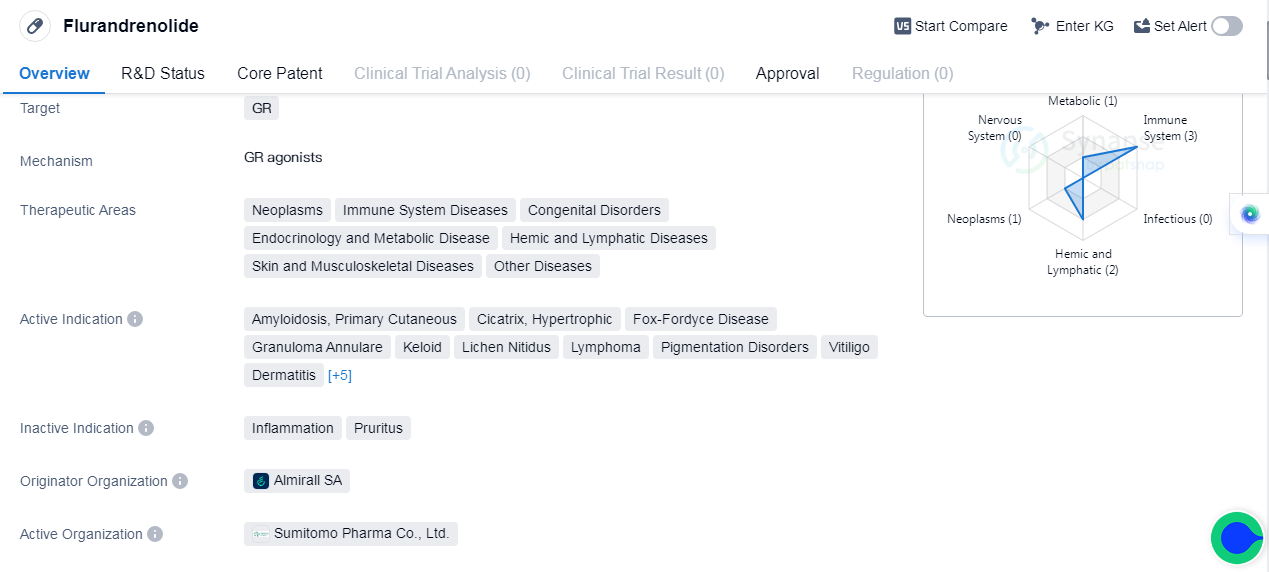
Flurandrenolide is a small molecule drug that targets the glucocorticoid receptor (GR). It has been approved for various therapeutic areas including neoplasms, immune system diseases, congenital disorders, endocrinology and metabolic disease, hemic and lymphatic diseases, skin and musculoskeletal diseases, and other diseases. The drug is indicated for the treatment of amyloidosis, primary cutaneous cicatrix, hypertrophic, Fox-Fordyce disease, granuloma annulare, keloid, lichen nitidus, lymphoma, pigmentation disorders, vitiligo, dermatitis, eczema, lichen planus, lupus erythematosus discoid, prurigo nodularis, and psoriasis.
Flurandrenolide was first approved in the United States in March 1963. It is developed by Almirall SA, a pharmaceutical company specializing in dermatology and respiratory diseases. The drug has reached the highest phase of development, which is approval, indicating its successful clinical trials and safety profile.
As a small molecule drug, Flurandrenolide is likely to have a well-defined chemical structure and can be easily synthesized. Its target, the glucocorticoid receptor, is involved in regulating various physiological processes, including immune response and inflammation. By targeting this receptor, Flurandrenolide may modulate these processes and provide therapeutic benefits in the indicated diseases.
The wide range of therapeutic areas and indications for Flurandrenolide suggests its versatility and potential efficacy in treating various medical conditions. It is particularly relevant in dermatology, where it can address skin disorders such as eczema, psoriasis, and dermatitis. Additionally, its use in neoplasms and immune system diseases indicates its potential in managing cancer and autoimmune conditions.
Being approved since 1963, Flurandrenolide has a long history of use and may have established safety and efficacy profiles. Its approval in the United States as the first country/location suggests its initial success and subsequent global adoption.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Flurandrenolide: GR agonists
From a biomedical perspective, GR agonists refer to substances or drugs that activate the glucocorticoid receptor (GR). The GR is a type of nuclear receptor found in cells that binds to the hormone cortisol or synthetic glucocorticoids. When activated by GR agonists, the receptor undergoes a conformational change and translocates into the nucleus of the cell. Once inside the nucleus, the GR can bind to specific DNA sequences called glucocorticoid response elements (GREs) and regulate the expression of target genes.
GR agonists are commonly used in clinical settings to mimic the effects of cortisol, a natural hormone produced by the adrenal glands. These agonists can have anti-inflammatory, immunosuppressive, and metabolic effects. They are used in the treatment of various conditions, such as autoimmune diseases, allergies, asthma, and certain types of cancer. By activating the GR, these agonists help to regulate the immune system, reduce inflammation, and modulate various physiological processes.
It is important to note that the use of GR agonists should be carefully monitored and controlled, as prolonged or excessive activation of the GR can lead to side effects such as immunosuppression, osteoporosis, and metabolic disturbances. Therefore, the dosage and duration of treatment with GR agonists should be determined by healthcare professionals based on the specific condition being treated.
Drug Target R&D Trends for Flurandrenolide
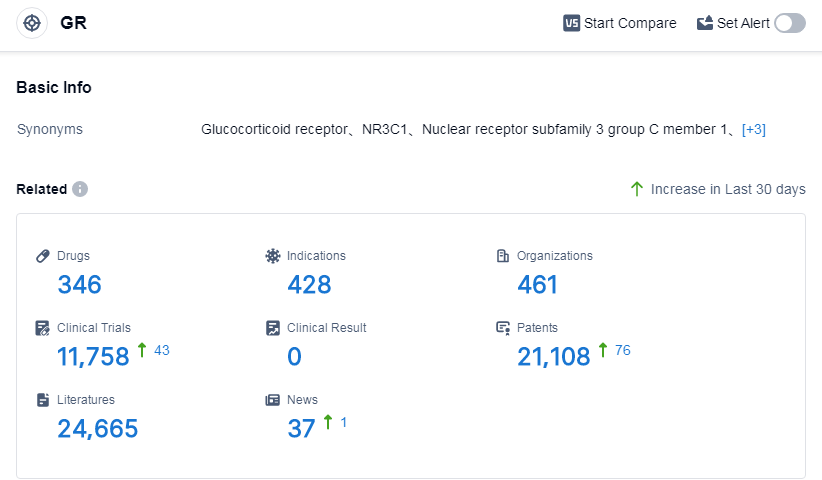
According to Patsnap Synapse, as of 2 Sep 2023, there are a total of 346 GR drugs worldwide, from 461 organizations, covering 428 indications, and conducting 11758 clinical trials.
The analysis of the target GR in the pharmaceutical industry reveals a dynamic and competitive landscape. Novartis AG emerges as the leading company with a robust R&D pipeline across multiple phases. Eczema, Asthma, and Psoriasis are the most approved indications, indicating significant progress in developing treatments for these conditions. Small molecule drugs dominate the development phases, reflecting the primary focus of research and development. The United States and China lead in terms of approved drugs, with China showing promising progress in the preclinical stage. Overall, the target GR presents a promising opportunity for companies and researchers to develop innovative therapies and address unmet medical needs in various therapeutic areas.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Flurandrenolide is a small molecule drug developed by Almirall SA that targets the glucocorticoid receptor. It has been approved for various therapeutic areas and indications, particularly in dermatology, neoplasms, and immune system diseases. Its long history of approval and broad range of indications highlight its potential as a versatile and effective treatment option.