Review of ALK Inhibitors for Lung Cancer
ALK (Anaplastic Lymphoma Kinase) is a receptor tyrosine kinase that plays a crucial role in various physiological processes in the human body. It is primarily involved in the development and maintenance of the nervous system, including the growth and survival of neurons. ALK is also implicated in the pathogenesis of certain cancers, such as anaplastic large cell lymphoma and non-small cell lung cancer (NSCLC), where it can become aberrantly activated.
The forms of ALK gene mutation include overexpression, fusion with other genes, and point mutation. The fusion of ALK genes was first discovered in lymphoma and was found in lung cancer patients for the first time in 2007. The fusion of the ALK gene with the Echinoderm Microtubule-associated protein-like 4 (EML4) gene is a crucial driver gene in NSCLC and an essential pathway in the formation and development of lung adenocarcinoma.
Big data shows that the proportion of ALK fusion mutations in lung cancer is 3% - 8%, with an incidence of about 5.6% in Chinese NSCLC. It's commonly seen in young, non-smoking/light smoking patients, lung adenocarcinoma (especially signet ring cell carcinoma) with a lack of other carcinogenic driver gene mutations, and is referred to as the "diamond mutation" compared to other lung cancer targets.
In recent years, with the continuous development of molecular biology, various ALK inhibitors have emerged one after another, presenting a "three generations in the same hall" drug use pattern. The efficacy is significant in patients with ALK fusion-positive NSCLC, greatly extending patients' survival time.
ALK Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 7 Sep 2023, there are a total of 71 ALK drugs worldwide, from 91 organizations, covering 60 indications, and conducting 575 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.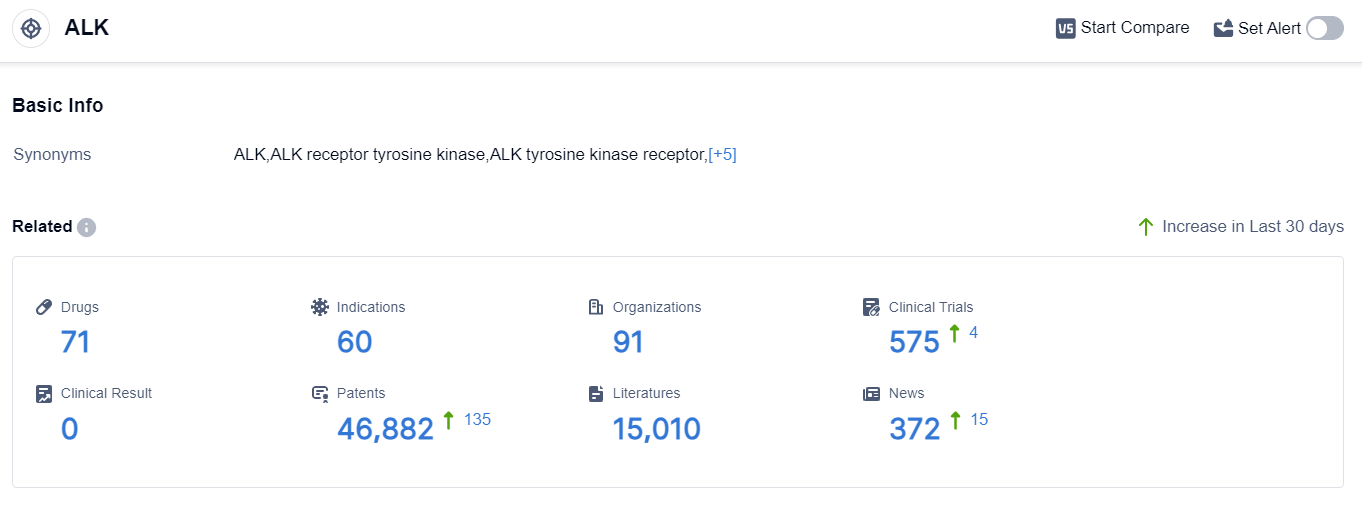
The analysis of target ALK reveals a competitive landscape with several companies making significant progress in the development of drugs. Roche Holding AG and Pfizer Inc. are leading in terms of the number of drugs in various stages of development.
ALK-targeted therapies have been approved for multiple indications, primarily in lung cancer and lymphoma. Small molecule drugs and PROTACs are the most rapidly progressing drug types, indicating intense competition and innovation in the field.
China, the United States, and the European Union are the key locations driving the development of ALK-targeted therapies. Overall, the target ALK shows great potential for the treatment of various cancers, and further research and development efforts are expected to drive future advancements in this field.
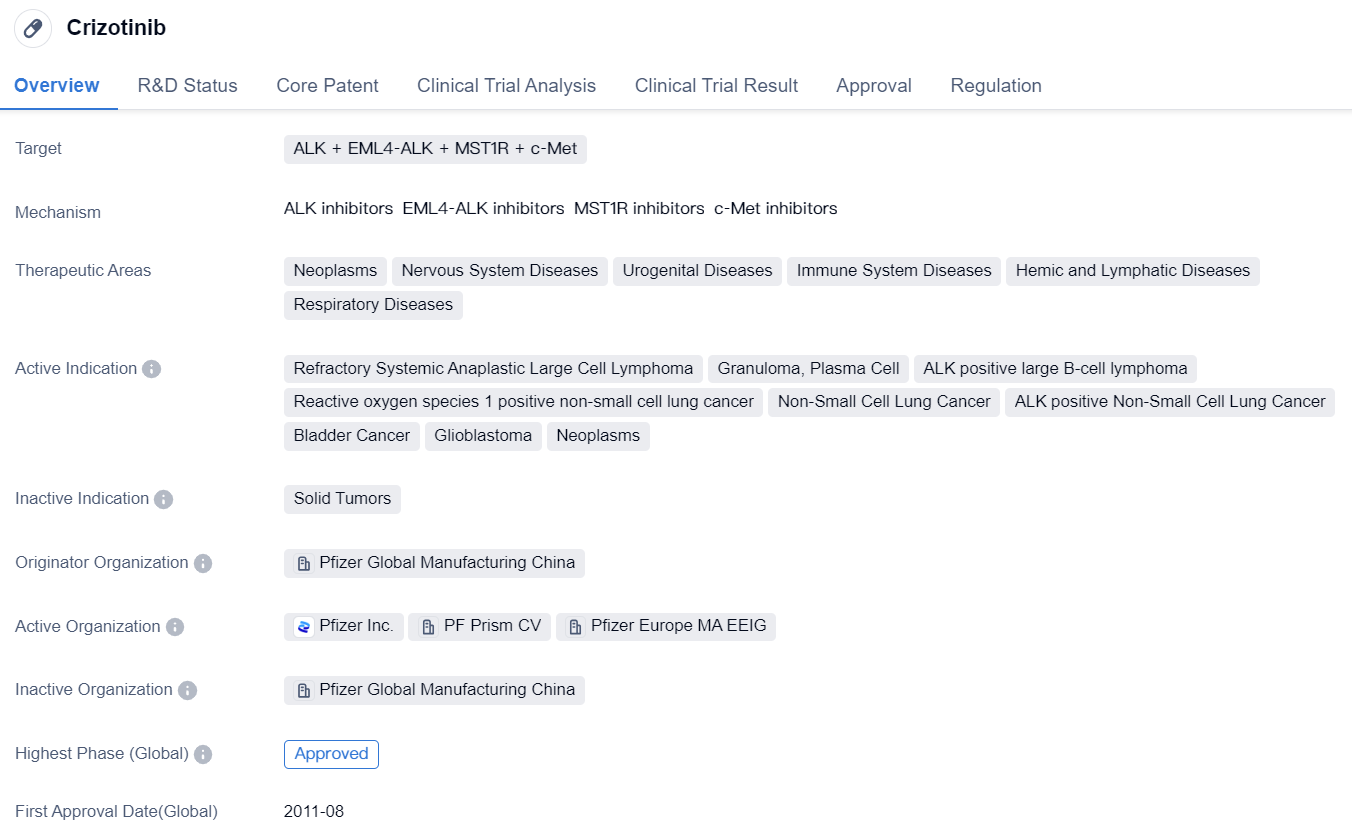
First Generation ALK Inhibitor: Crizotinib
Crizotinib is the first globally approved ALK-targeted drug and first-generation ALK inhibitor, which has completely changed the treatment landscape for ALK-positive NSCLC. Crizotinib is an orally administered small molecule inhibitor that targets ALK, ROS1, and c-MET tyrosine kinases. In Phase I and II studies, Crizotinib provided prolonged relief for patients with advanced ALK-positive NSCLC. In the phase III PROFILE 1007 study, compared to chemotherapy, frontline Crizotinib showed superior progression-free survival (PFS) benefit in patients who had received prior treatment.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
However, the pharmacokinetic failure of Crizotinib is mainly due to poor blood-brain barrier penetration, and the central nervous system (CNS) is a common site of Crizotinib progression. Patients receiving Crizotinib treatment can develop acquired drug resistance, with L1196M, G1269A, and C1156Y mutations altering the structure of the ATP binding pocket, thus preventing Crizotinib from binding with ALK. After Crizotinib resistance, the second-generation ALK inhibitors have emerged.
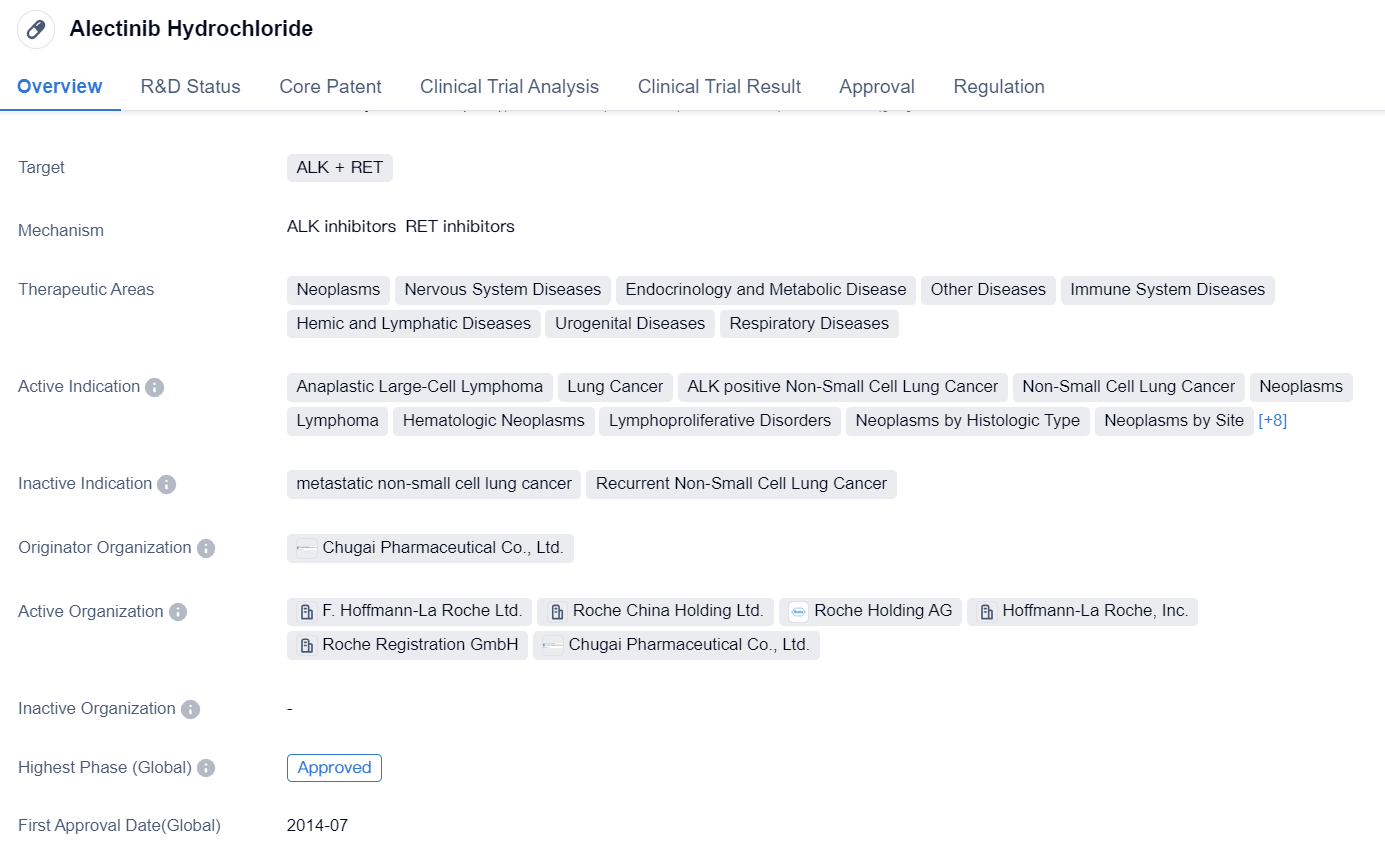
Second-generation ALK inhibitor: Alectinib Hydrochloride
Alectinib Hydrochloride is a highly selective second-generation inhibitor for ALK, compared to Crizotinib, Alectinib Hydrochloride is not a P-glycoprotein substrate, and has better permeability to the blood-brain barrier. Based on two single-arm trials (NP28761 and NP28673), the FDA approved Alectinib Hydrochloride for second-line treatment of ALK-positive NSCLC in 2015. The J-ALEX trial was the first study to show that Alectinib Hydrochloride has PFS superiority and is better tolerated than Crizotinib at a daily dose of 300mg twice. Based on the phase III ALEX study of Alectinib Hydrochloride 600mg twice daily, FDA approved Alectinib Hydrochloride for first-line treatment of ALK-positive NSCLC in 2017.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
A prospective real-world study investigated the strategy of switching to Alectinib Hydrochloride in ALK-positive NSCLC patients who had no disease progression under initial Crizotinib treatment. The research findings suggest that an early transition from Crizotinib to Alectinib Hydrochloride may be a viable option and may promote better treatment compliance.
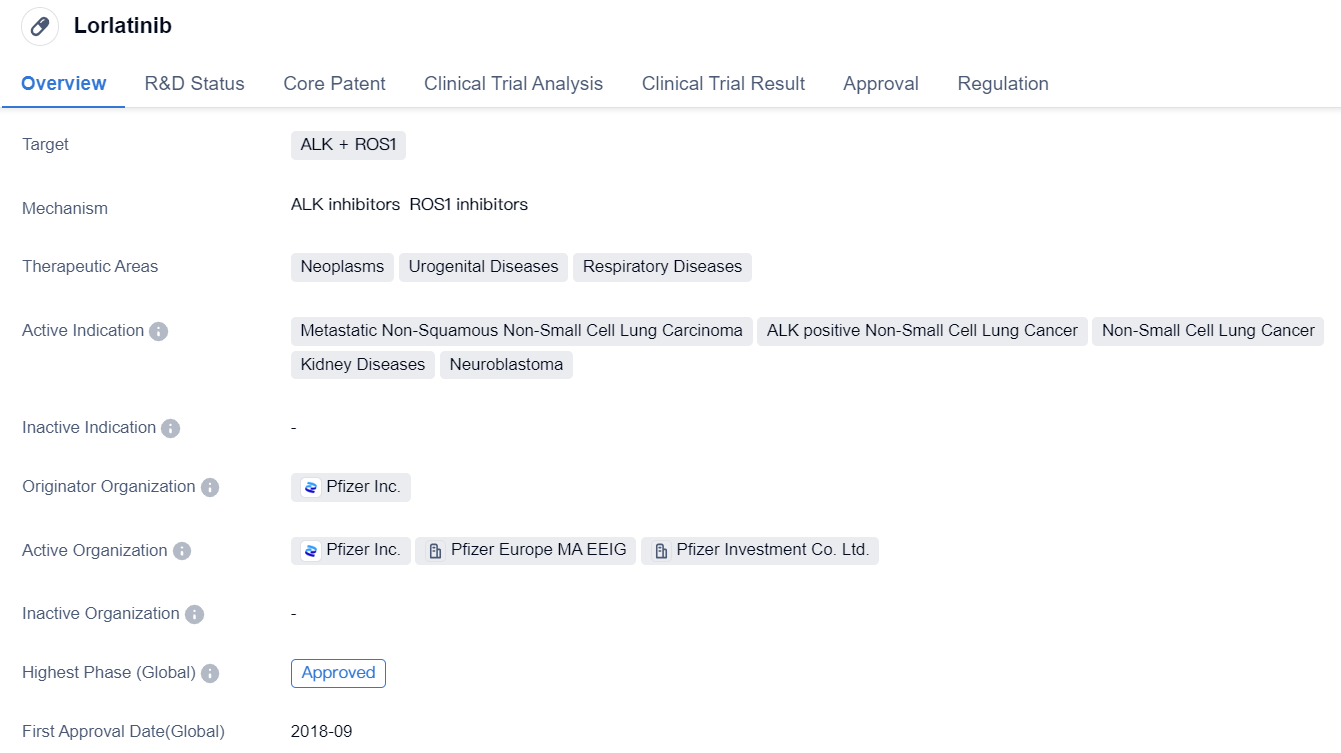
Third-generation ALK inhibitor: Lorlatinib
Lorlatinib is a third-generation ALK/ROS1 dual-target inhibitor, specifically designed to penetrate the blood-brain barrier and inhibit ALK resistant mutations. It has been optimized based on the issues of the first and second-generation ALK inhibitors and adopts a completely different small molecule macrocyclic amide structure. On the one hand, it binds more strongly and stably to the kinase domain, enhancing resistance to drug resistance; on the other hand, it has strong blood-brain barrier penetration ability, strong brain entry effect, and can effectively target brain metastasis in ALK mutated patients. At the 2022 AACR Annual Meeting, the latest research data from the Phase III CROWN study was unveiled. Based on these study results, in April 2022, the NMPA approved Lorlatinib monotherapy for local advanced or metastatic NSCLC patients with ALK-positivity.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The CROWN study shows that, after a median follow-up of 3 years, Lorlatinib continues to demonstrate a significant improvement over Crizotinib in the primary endpoint, the progression-free survival (PFS) evaluated by independent central review (BICR) (HR=0.27; 95%CI: 0.18-0.39). The three-year PFS rate is as high as 63.5%, equivalent to a 73% reduction in the risk of disease progression or death.