A Comprehensive Review of Lubiprostone's R&D Innovations and Drug Target Mechanism
Lubiprostone's R&D Progress
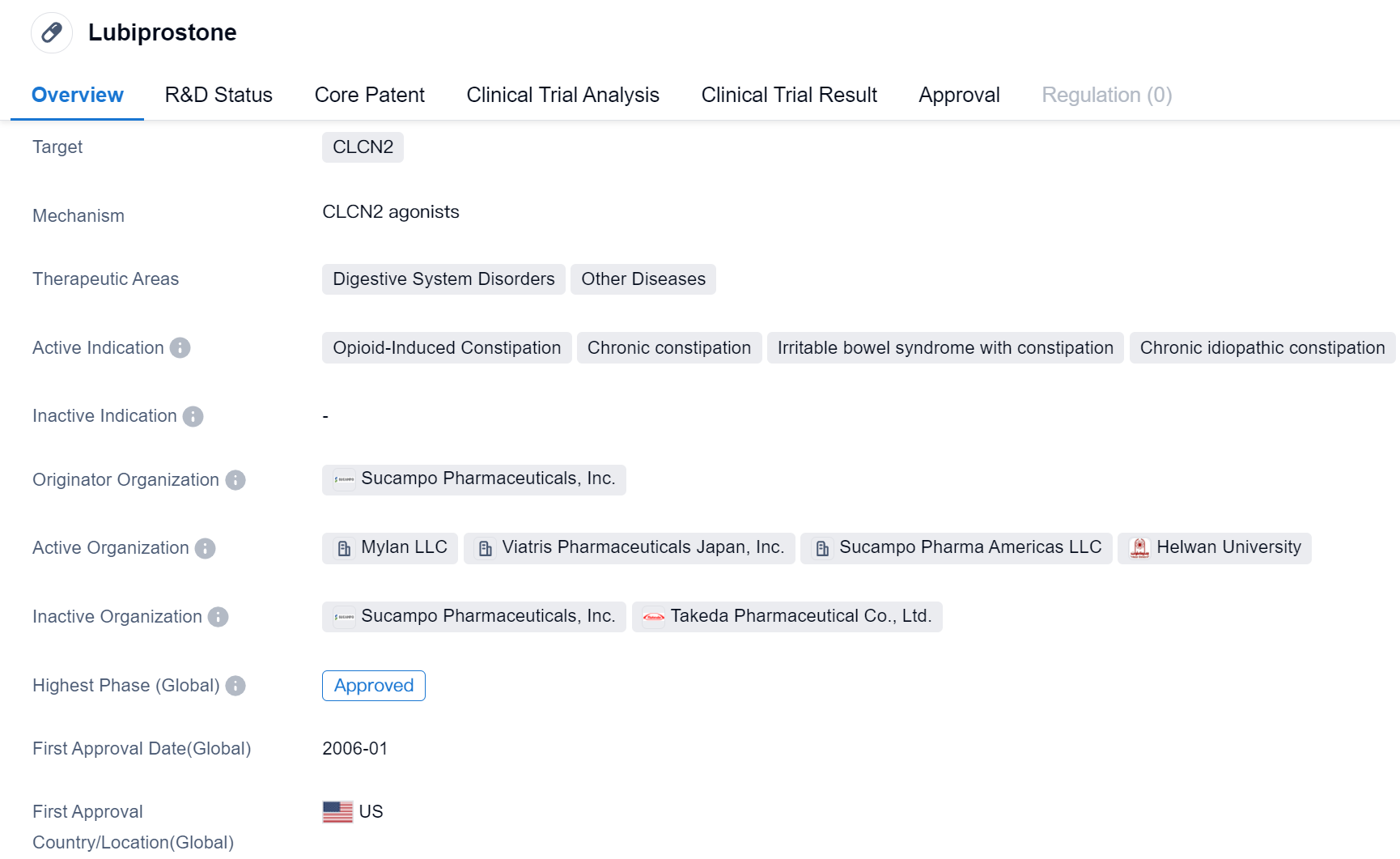
Lubiprostone is a small molecule drug th1at targets CLCN2 and is primarily used in the treatment of digestive system disorders and other diseases. It has been approved for various indications, including opioid-induced constipation, chronic constipation, irritable bowel syndrome with constipation, chronic idiopathic constipation, and non-alcoholic fatty liver disease.
The drug was developed by Sucampo Pharmaceuticals, Inc., an originator organization in the pharmaceutical industry. It received its first approval in the United States in January 2006.
Lubiprostone's mechanism of action involves activating specific chloride channels in the gastrointestinal tract, which helps to increase fluid secretion and improve bowel movements. This makes it particularly effective in treating conditions characterized by constipation.
Opioid-induced constipation is a common side effect of opioid pain medications, and Lubiprostone has shown efficacy in alleviating this symptom. Chronic constipation, irritable bowel syndrome with constipation, and chronic idiopathic constipation are conditions that can significantly impact a patient's quality of life, and Lubiprostone offers a potential treatment option for these individuals.
Additionally, Lubiprostone has been investigated for its potential use in non-alcoholic fatty liver disease, a condition characterized by the accumulation of fat in the liver. While further research is needed to establish its efficacy in this area, the drug shows promise as a potential therapeutic option.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Lubiprostone: CLCN2 agonists
CLCN2 agonists are a type of drug that specifically target and activate the CLCN2 ion channel. From a biomedical perspective, CLCN2 refers to the chloride channel 2, which is a protein involved in the transport of chloride ions across cell membranes. Agonists are substances that bind to a specific receptor or channel and activate it, leading to an increase in its activity.
In the context of biomedicine, CLCN2 agonists would be compounds or drugs that bind to the CLCN2 ion channel and enhance its function. These agonists can modulate chloride ion transport, which has implications in various physiological processes and diseases. For example, CLCN2 agonists might be explored as potential therapeutic agents for conditions like epilepsy, where abnormal chloride ion transport is implicated.
It's important to note that without further context, it is challenging to provide a more specific explanation or discuss the clinical relevance or potential side effects of CLCN2 agonists.
Drug Target R&D Trends for Lubiprostone
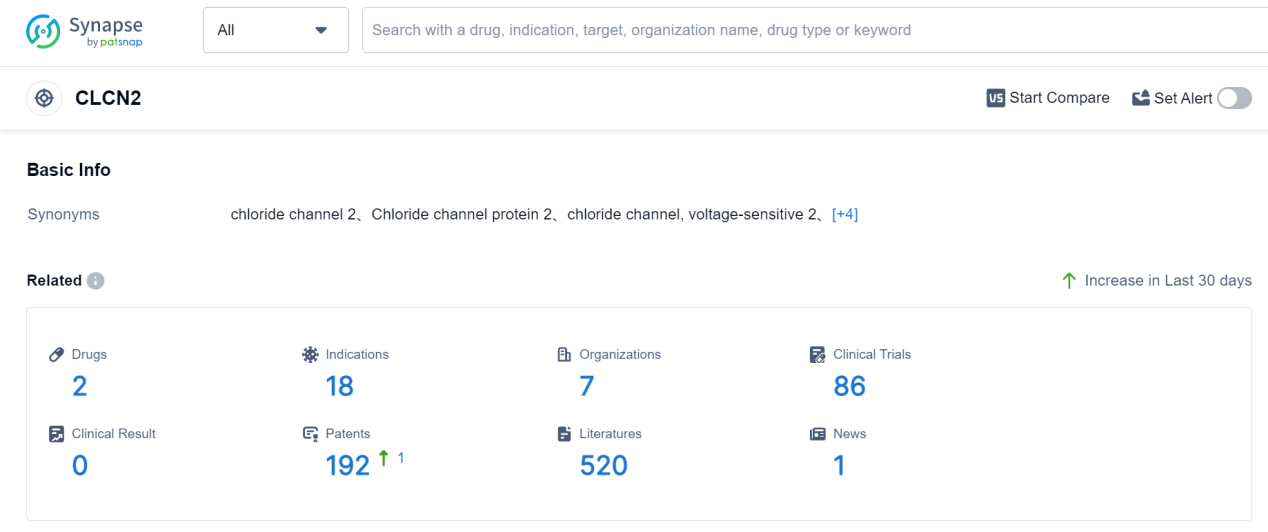
CLCN2 is a gene that encodes for a chloride channel protein called CLC-2, which is predominantly expressed in the brain and spinal cord. This channel plays a crucial role in maintaining the electrical balance of neurons and regulating the movement of chloride ions across cell membranes. CLCN2 is involved in various physiological processes, including the control of neuronal excitability, synaptic transmission, and cerebrospinal fluid production. Dysregulation of CLCN2 has been associated with neurological disorders such as epilepsy and migraine. Understanding the role of CLCN2 in the human body can provide valuable insights for the development of novel therapeutic strategies targeting these conditions.
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 2 CLCN2 drugs worldwide, from 7 organizations, covering 18 indications, and conducting 86 clinical trials.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Lubiprostone is a small molecule drug that targets CLCN2 and has been approved for the treatment of various digestive system disorders and other diseases. Its approval in the United States in 2006 marked an important milestone in the pharmaceutical industry, providing healthcare professionals with a valuable tool in managing conditions such as opioid-induced constipation, chronic constipation, irritable bowel syndrome with constipation, chronic idiopathic constipation, and potentially non-alcoholic fatty liver disease.